110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在晚期实体瘤患者中阿帕替尼对硝苯地平和华法林药代动力学的影响
Authors Zhu YT, Teng Z, Zhang YF, Li W, Guo LX, Liu YP, Qu XJ, Wang QR, Mao SY, Chen XY, Zhong DF
Received 5 November 2019
Accepted for publication 27 April 2020
Published 20 May 2020 Volume 2020:14 Pages 1963—1970
DOI https://doi.org/10.2147/DDDT.S237301
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Yan Zhu
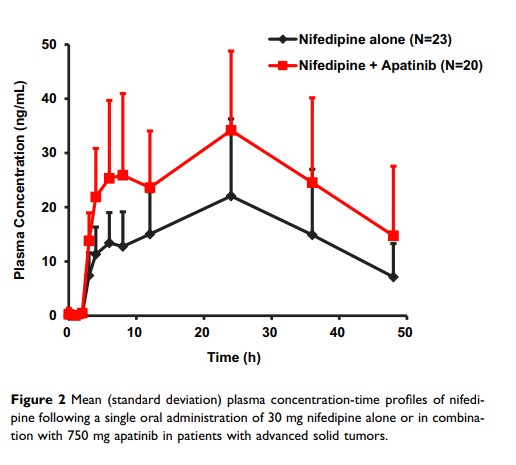
Background and Purpose: Apatinib is a small-molecule tyrosine kinase inhibitor for the treatment of recurrent or progressive advanced-stage gastric adenocarcinoma or gastroesophageal junction cancer. The in vitro inhibition studies suggested that apatinib exerted potent inhibition on CYP3A4 and CYP2C9. To evaluate the potential of apatinib as a perpetrator in CYP450-based drug–drug interactions in vivo, nifedipine and warfarin were, respectively, selected in the present study as the probe substrates of CYP3A4 and CYP2C9 for clinical drug–drug interaction studies. Since hypertension and thrombus are common adverse effects of vascular targeting anticancer agents, nifedipine and warfarin are usually coadministered with apatinib in clinical practice.
Methods: A single-center, open-label, single-arm, and self-controlled trial was conducted in patients with advanced solid tumors. The patients received a single dose of 30 mg nifedipine on Day 1/14 and a single dose of 3 mg warfarin on Day 3/16. On Day 9– 21, the subjects received a daily dose of 750 mg apatinib, respectively. The pharmacokinetics of nifedipine and warfarin in the absence or presence of apatinib was, respectively, investigated.
Results: Compared with the single oral administration, coadministration with apatinib contributed to the significant increases of AUC0– 48h and Cmax of nifedipine by 83% (90% confidence interval [CI] 1.46– 2.31) and 64% (90% CI 1.34– 2.01), respectively. Similarly, coadministration with apatinib contributed to the significant increases of AUC0-t and Cmax of S-warfarin by 92% (90% CI 1.68– 2.18) and 24% (90% CI 1.10– 1.39), respectively.
Conclusion: Concomitant apatinib administration resulted in significant increases in systemic exposure to nifedipine and S-warfarin. Owing to the risk of pharmacokinetic drug–drug interactions based on CYP3A4/CYP2C9 inhibition by apatinib, caution is advised in the concurrent use of apatinib with either CYP2C9 or CYP3A4 substrates.
Keywords: apatinib, drug-drug interaction, CYP3A4, CYP2C9, nifedipine, warfarin