110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

化疗加安罗替尼与单独化疗作为晚期非小细胞肺癌的二线或三线抢救治疗的安全性和有效性试验
Authors Wang H, Chu J, Zhao Y, Tang H, Wang L, Zhou M, Yan Z, Liu Y, Yao Z
Received 13 February 2020
Accepted for publication 9 May 2020
Published 22 May 2020 Volume 2020:12 Pages 3827—3834
DOI https://doi.org/10.2147/CMAR.S249678
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Purpose: Anlotinib is a newly developed oral multitarget tyrosine kinase inhibitor. We retrospectively evaluated the toxicity and clinical efficacy of chemotherapy combined with anlotinib versus chemotherapy alone for metastatic/advanced non-small cell lung cancer (NSCLC) in patients who failed first- or second-line systemic treatment in China.
Patients and Methods: In this retrospective trial, ninety-four advanced NSCLC patients received chemotherapy combined with anlotinib (n = 41) or chemotherapy alone (n = 53) in Henan Cancer Hospital. We recorded the objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS) and adverse events (AEs).
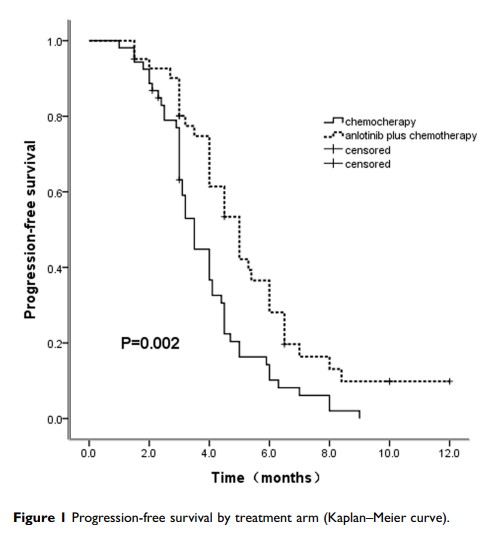
Results: In the anlotinib plus chemotherapy group, eleven patients (27%) achieved a PR (partial response), and twenty-one patients (51%) achieved SD (stable disease), with an ORR of 27% and a DCR of 78%. In the chemotherapy alone group, eight patients (15%) achieved a PR, and nineteen patients (36%) had SD, with an ORR of 15% and a DCR of 51%. The ORR in the combination arm was slightly, but not obviously, higher than that in the chemotherapy arm (27% vs 15%, p > 0.05). In addition, the DCR was significantly higher in the combination arm than in the chemotherapy alone arm (78% vs 51%, p=0.007). At the end of follow-up, patients in the combination arm had a 1.5-month longer median PFS than patients in the chemotherapy arm; this difference was statistically significant (5.0 vs 3.5, p=0.002). The median OS was not achieved at the final analysis. The hematological and nonhematological toxicities were well tolerated and controlled. In general, most toxicity was limited to grade I or II, well tolerated and controlled.
Conclusion: Our study suggests that anlotinib combined with chemotherapy may be an effective and well-tolerated treatment for advanced NSCLC in patients who fail first- or second-line therapy.
Keywords: anlotinib, chemotherapy, toxicity, efficacy, advanced non-small cell lung cancer