108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Brevilin A,一种天然的倍半萜内酯,通过 Akt/mTOR 和 STAT3 信号通路抑制三阴性乳腺癌细胞的生长
Authors Qu Z, Lin Y, Mok DKW, Bian Q, Tai WCS, Chen S
Received 4 April 2020
Accepted for publication 21 May 2020
Published 10 June 2020 Volume 2020:13 Pages 5363—5373
DOI https://doi.org/10.2147/OTT.S256833
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Purpose: Triple-negative breast cancer (TNBC) is a a breast cancer subtype characterized by a lack of estrogen receptor, progesterone receptor and human epidermal growth receptor 2 and is associated with poorer prognoses when compared to other breast cancers. Thus, novel anti-cancer agents with high efficacy are urgently needed. Brevilin A (BA), a natural sesquiterpene lactone, has been reported to exhibit anti-cancer effects. However, the effects of BA on TNBC have not yet been demonstrated. In this study, we investigated the anti-TNBC effects and the underlying mechanism of BA, in vitro and in vivo.
Methods: Two TNBC cell lines and a xenograft mouse model were employed to assess the effects of BA. Cell viability was detected by MTT assay. Cell cycle status and apoptosis were evaluated by flow cytometry. Cell migration was measured by wound-healing assay. Protein expression was measured by Western blotting analysis. The in vivo anti-cancer activity of BA was assessed in orthotopic tumor xenograft mice.
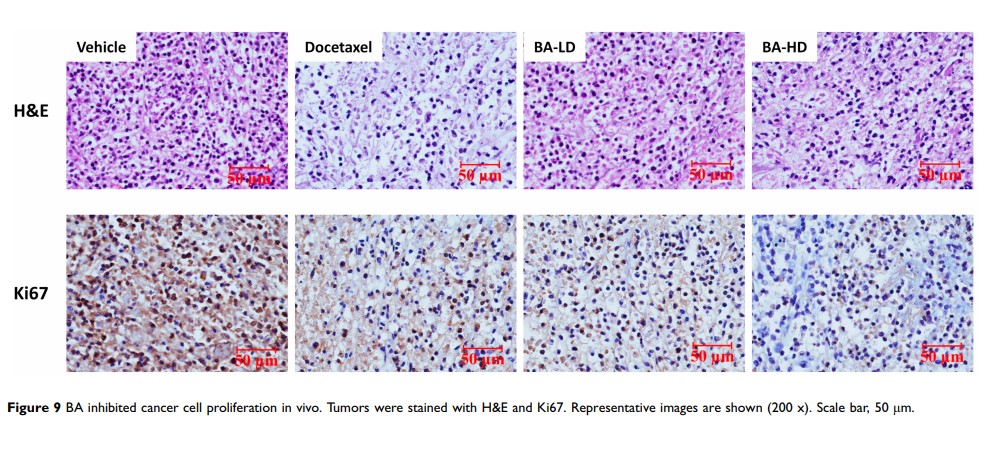
Results: BA significantly inhibited the growth of TNBC cells in a dose- and time-dependent manner via induction of cell cycle arrest at G2/M phase arrest and apoptosis. BA also inhibited tumor cell migration. BA significantly downregulated the expression of Akt, mTOR, Stat3 and their phosphorylation, and thus inhibiting the activation of the Akt/mTOR and STAT3 signaling pathways. Furthermore, oral administration of BA at 25 or 50 mg/kg leads to significant inhibition of tumor growth and proliferation in tumor xenograft model mice.
Conclusion: BA significantly inhibited the growth and migration of TNBC cells, and induced cell cycle arrest and apoptosis. These inhibitory effects were associated with the suppression of the Akt/mTOR and Stat3 signal pathways. Based on our findings, BA possesses a promising candidate for development as an anti-cancer therapeutic drug against TNBC.
Keywords: anticancer, apoptosis, cell cycle arrest, migration, cancer therapy