110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

HMGA1 通过 Wnt/β-连环蛋白信号通路调节 GIST 患者循环肿瘤细胞的干细胞样特性
Authors Chen M, Xu K, Li B, Wang N, Zhang Q, Chen L, Zhang D, Yang L, Xu Z, Xu H
Received 10 February 2020
Accepted for publication 6 April 2020
Published 11 June 2020 Volume 2020:13 Pages 4943—4956
DOI https://doi.org/10.2147/OTT.S249063
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
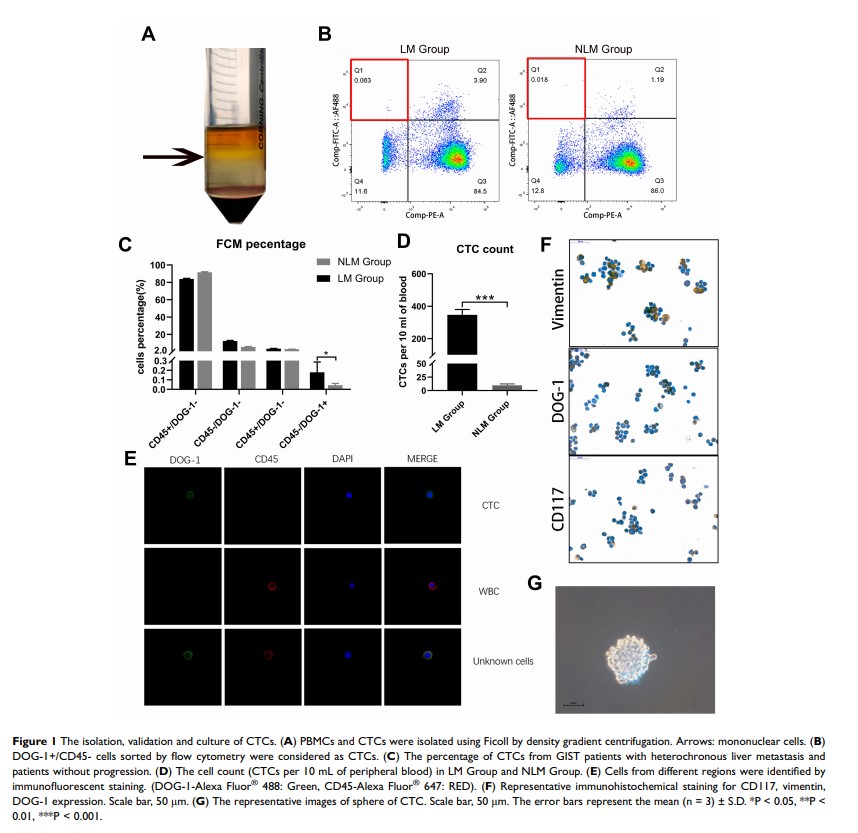
Background: Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the digestive system. Circulating tumor cells (CTCs) have been proven to be critical in the recurrence and metastasis of diseases; however, the characteristics of CTCs of GIST are still unclear.
Methods: We sorted out and verified the validity of CTCs from peripheral blood of gastrointestinal stromal tumor (GIST) patients with or without heterochronous liver metastasis using flow cytometry (FCM). Differential genes were analyzed between the GIST patients with and without liver metastasis using next-generation sequencing (NGS).
Results: The preliminary study on the characteristics of CTCs revealed that CTCs of GIST patients with heterochronous liver metastasis had stronger stem cell-like properties (SC-like properties) than CTCs of those without liver metastasis. Furthermore, NGS followed with a series of assays revealed that HMGA1 played a critical role in regulating the SC-like properties of CTCs. Mechanistically, HMGA1 could activate Wnt/β-catenin pathway in vitro and vivo. Moreover, we found that the expression level of HMGA1 in CTCs was an independent risk factor probably influencing the prognosis of GIST patients.
Conclusion: Our findings indicate the significant role of HMGA1 in SC-like properties, IM resistance and eventually hepatic metastasis formation of CTCs. Targeting HMGA1 in CTCs may be a therapeutic strategy for GIST patients with hepatic metastasis.
Keywords: circulating tumor cells, gastrointestinal stromal tumor, stem cell-like properties, heterochronous liver metastasis, HMGA1, Wnt/β-catenin