110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

埃克替尼作为伴有 EGFR 突变的 II-IIIA 期肺腺癌患者的辅助治疗(ICWIP 研究):随机对照试验的研究方案
Authors Liu YT, Hao XZ, Liu DR, Cheng G, Zhang SC, Xiao WH, Hu Y, Liu JF, He M, Ding CM, Zhang L, Wang J, Li H, Dong GL, Zhi XY, Li J, Shi YK
Received 27 November 2019
Accepted for publication 21 April 2020
Published 17 June 2020 Volume 2020:12 Pages 4633—4643
DOI https://doi.org/10.2147/CMAR.S240275
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
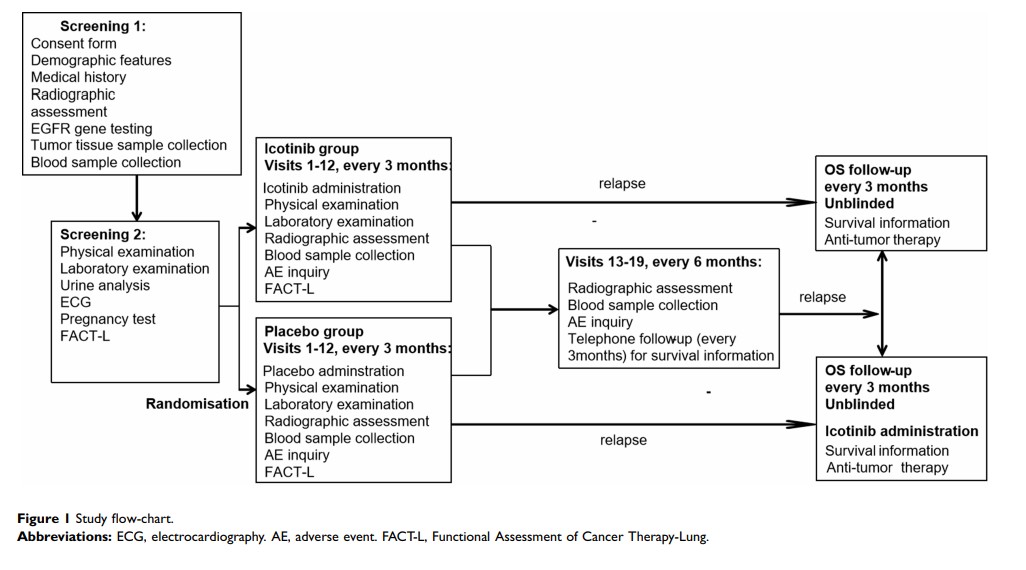
Abstract: The efficacy and possible role of epidermal growth factor receptor tyrosine kinase inhibitors in treating early-stage non-small-cell lung cancer have yet to be established. Therefore, we aimed to explore the efficacy and safety of icotinib in completely resected EGFR-mutant stage II–IIIA lung adenocarcinoma patients who underwent standard chemotherapy. This is a randomised, double-blinded, placebo-controlled, multicentre, Phase III trial. A total of 124 patients aged 18– 75 years who qualified the inclusion criteria were recruited. These patients were randomised (1:1) to receive either icotinib (125 mg 3 times per day) or placebo (the same dosage and frequency) for 36 months, followed by a further 36 months of observational window. The primary endpoint is disease-free survival (DFS), while the secondary endpoints are overall survival, 3-year and 5-year DFS, safety and tolerability of the medication, and health-related quality-of-life. Analyses will be conducted in a full analysis set and a per-protocol set as well. To our knowledge, the present study is the first randomised, double-blinded, placebo-controlled, multicenter trial designed to explore efficacy and safety of icotonib in this population. The results obtained in the near future may provide potential guidance in clinical practice.
Trial Registration: This trial was registered on www.ClinicalTrail.gov as NCT02125240.
Keywords: EGFR mutation, non-small-cell lung cancer, lung adenocarcinoma, icotinib, adjuvant chemotherapy