110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

定量蛋白质组学分析表明,lncRNA HULC 的上调促进胶质母细胞瘤细胞的发病机理
Authors Hu Y, Ye S, Li Q, Yin T, Wu J, He J
Received 7 March 2020
Accepted for publication 15 May 2020
Published 22 June 2020 Volume 2020:13 Pages 5927—5938
DOI https://doi.org/10.2147/OTT.S252915
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Purpose: Glioblastoma (GBM) is an aggressive central nervous system (CNS) cancer and a serious threat to human health. The long noncoding RNA (lncRNA) HULC has been implicated in GBM, but the molecular mechanism is uncertain. This study used quantitative proteomic analysis for global identification of HULC-regulated proteins in glioblastoma cells and identification of potential biomarkers.
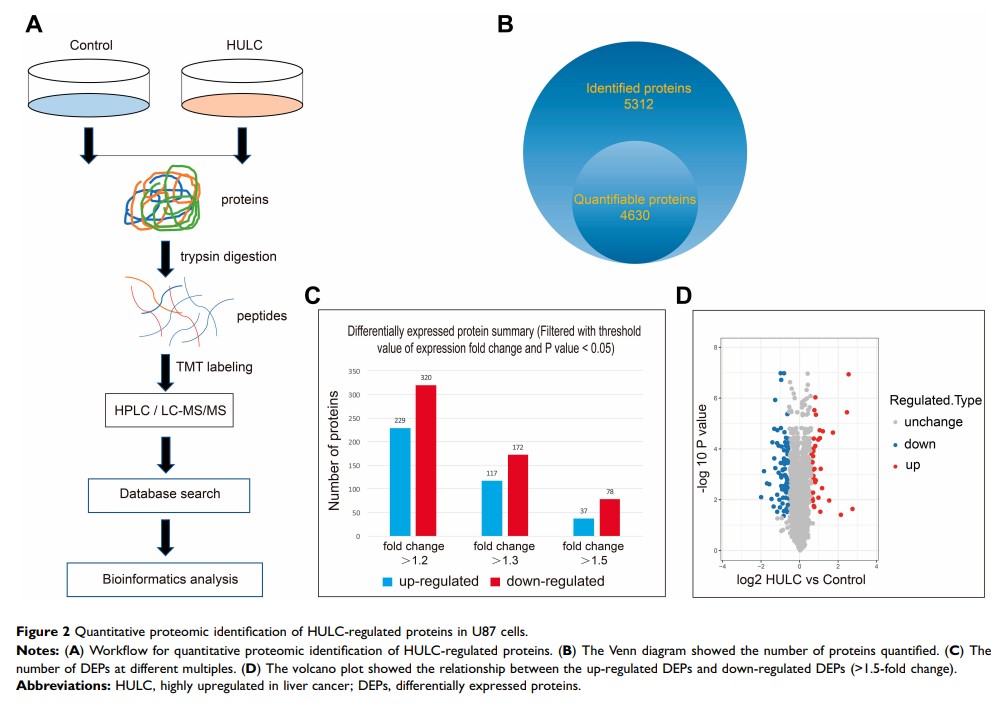
Materials and Methods: qRT-PCR was used to determine the expression of HULC in U87 cells stably transfected with HULC or an empty vector (control). The CCK-8 assay, transwell assay, and wound-scratch assay were used to measure cell proliferation, invasion, and migration. Quantitative proteomics using Tandem Mass Tag (TMT) labeling, high-performance liquid chromatography (HPLC) fractionation, and liquid chromatography–mass spectrometry (LC-MS/MS) analysis were used to identify differentially expressed proteins (DEPs). Screened proteins were validated by parallel reaction monitoring (PRM) and Western blotting.
Results: Overexpression of HULC led to increased cell proliferation, invasion, and migration. HULC overexpression also led to significant upregulation of 37 proteins and downregulation of 78 proteins. Bioinformatics analysis indicated these proteins had roles in cellular component, biological process, and molecular function. PRM results of 8 of these proteins (PTK2, TNC, ITGAV, LASP1, MAPK14, ITGA1, GNA13, RRAS) were consistent with the LC-MS/MS and Western blotting results.
Conclusion: The results of present study suggest that lncRNA HULC promotes GBM cell proliferation, invasion, and migration by regulating RRAS expression, suggesting that RRAS may be a potential biomarker or therapeutic target for this cancer.
Keywords: glioblastoma, LncRNA HULC, quantitative proteomics, PRM