110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

基于 β-葡萄糖苷酶/苦杏仁苷的磁定向酶/前药用于前列腺癌治疗
Authors Zhou J, Hou J, Rao J, Zhou C, Liu Y, Gao W
Received 14 December 2019
Accepted for publication 9 June 2020
Published 29 June 2020 Volume 2020:15 Pages 4639—4657
DOI https://doi.org/10.2147/IJN.S242359
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Background: β-Glucosidase (β-Glu) can activate amygdalin to kill prostate cancer cells, but the poor specificity of this killing effect may cause severe general toxicity in vivo, limiting the practical clinical application of this approach.
Materials and Methods: In this study, starch-coated magnetic nanoparticles (MNPs) were successively conjugated with β-Glu and polyethylene glycol (PEG) by chemical coupling methods. Cell experiments were used to confirm the effects of immobilized β-Glu on amygdalin-mediated prostate cancer cell death in vitro. Subcutaneous xenograft models were used to carry out the targeting experiment and magnetically directed enzyme/prodrug therapy (MDEPT) experiment in vivo.
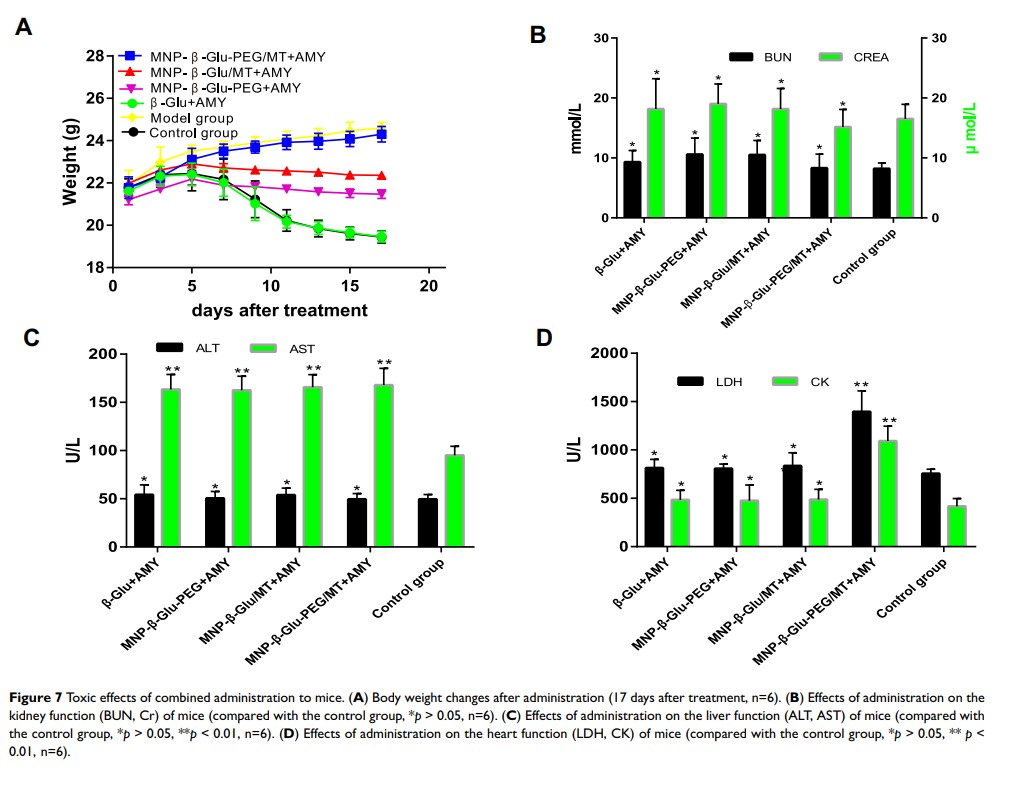
Results: Immobilized β-Glu activated amygdalin-mediated prostate cancer cell death. Tumor-targeting studies showed that PEG modification increased the accumulation of β-Glu-loaded nanoparticles in targeted tumor tissue subjected to an external magnetic field and decreased the accumulation of the nanoparticles in the liver and spleen. Based on an enzyme activity of up to 134.89 ± 14.18mU/g tissue in the targeted tumor tissue, PEG-β-Glu-MNP/amygdalin combination therapy achieved targeted activation of amygdalin and tumor growth inhibition in C57BL/6 mice bearing RM1 xenografts. Safety evaluations showed that this strategy had some impact on liver and heart function but did not cause obvious organ damage.
Conclusion: All findings indicate that this magnetically directed enzyme/prodrug therapy strategy has the potential to become a promising new approach for targeted therapy of prostate cancer.
Keywords: magnetic nanoparticles, β-glucosidase, amygdalin, prostate cancer, magnetically directed enzyme/prodrug therapy