110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

基于真实世界的经验,对氟维司群与依维莫司加依西美坦用于绝经后转移性乳腺癌对芳香化酶抑制剂耐药治疗的治疗模式和结果的比较
Authors Li Y, Xie Y, Gong C, Zhao Y, Zhang J, Zhang S, Wang L, Chen S, Hu X, Wang B
Received 31 March 2020
Accepted for publication 12 June 2020
Published 30 June 2020 Volume 2020:16 Pages 607—615
DOI https://doi.org/10.2147/TCRM.S255365
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Background: Fulvestrant (FUL) and the combination of everolimus and exemestane (EVE-EXE) were the options to treat hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) patients who were refractory to aromatase inhibitors (AIs). The practical knowledge of treatment patterns and outcomes between the two regimens is essential for improving treatment decisions.
Methods: HR+/HER2− MBC patients, who were refractory to AI, were treated with FUL or EVE-EXE from June 2013 to June 2016 were included. Treatment patterns, progression-free survival (PFS), overall survival (OS), and toxicity were reported. Propensity score matching (PSM) was used to minimize potential confounders.
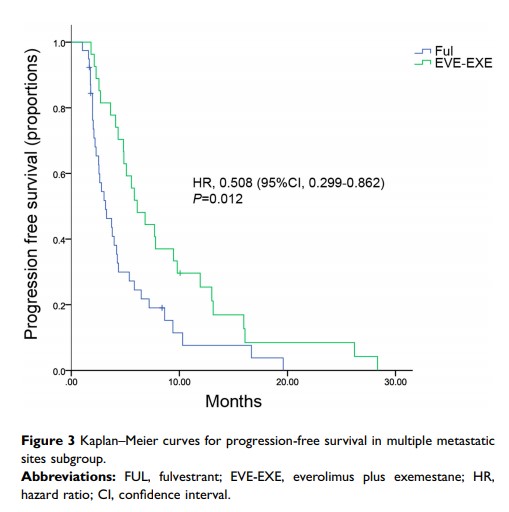
Results: A total of 168 patients were enrolled. Of 168 patients, 124 patients were treated with FUL and 44 patients received EVE-EXE. Patients who were treated with EVE-EXE were younger, more likely to have visceral, liver, multiple sites of metastases, and had received more prior chemotherapy. After adjusting for propensity score matching (PSM), no significant difference in PFS was found between two groups (P =0.419). However, in the subgroup of multiple metastatic sites, the median PFS was significantly improved in the EVE-EXE arm compared with FUL arm (6.1 vs 3.2 months, respectively, P =0.012). More patients in EVE-EXE arm discontinued treatment due to adverse events than in the FUL arm.
Conclusion: A substantial difference in treatment patterns was observed between the two arms. Clinical outcomes were comparable after PSM.
Clinicaltrials.gov Identifier: NCT03695341 (May 14, 2018).
Keywords: fulvestrant, everolimus, exemestane, metastatic breast cancer, endocrine therapy