110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

两种格列美脲片 1-mg 制剂在中国受试者体内的生物等效性和药代动力学参数的评估
Authors Ju G, Yan K, Xu Y, Chen S, Zheng Z, Qiu W
Received 12 February 2020
Accepted for publication 9 June 2020
Published 6 July 2020 Volume 2020:14 Pages 2637—2644
DOI https://doi.org/10.2147/DDDT.S249355
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
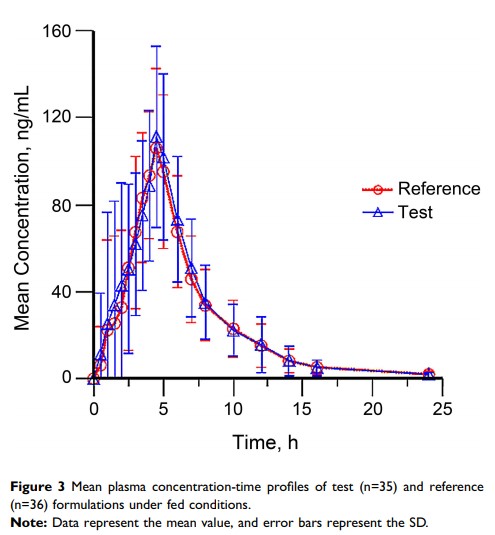
Purpose: Glimepiride, an FDA-approved oral hypoglycemic drug, is a long-acting sulfonylurea (SU), used for treating type 2 diabetes. The study aimed to evaluate the bioequivalence and safety profiles of two different formulations of glimepiride 1 mg from two different manufactures in healthy Chinese subjects in the fasting and fed state in order to acquire adequate pharmacokinetic evidence for registration approval of the test formulation.
Patients and Methods: This study is an open-label, two-period, two-sequence, randomized, two-way crossover pharmacokinetic study in healthy Chinese subjects in the fasting and fed state. Seventy-two subjects were randomly assigned to the fasting group and the fed group (n=36 each). We collected blood samples, 24-h post drug administration. The plasma concentration of glimepiride was assessed using HPLC coupled with mass spectrometry. The following parameters were evaluated: AUC0-inf, AUC0-last, Cmax, t1⁄2, Tmax, and λz. Safety was determined based on the occurrence of adverse events (AEs) and laboratory examinations (biochemistry, hematology, and urinalysis) throughout the entire study period.
Results: The geometric mean ratios (GMR) amongst the two glimepiride formulations for the primary pharmacokinetic parameters, ie, AUC0-inf, AUC0-last, and Cmax as well as the corresponding 90% CIs, were all within the range of 80.00– 125.00% in the fasting and fed state. The safety profile for both treatments was comparable.
Conclusion: PK analysis revealed that the test and reference formulations of glimepiride were bioequivalent and well tolerated in healthy Chinese subjects. Chinese Clinical Trials Registry identifier: CTR20171121.
Clinical Trial Registration Number: CTR20171121.
Keywords: glimepiride, bioequivalence, pharmacokinetics, HPLC-MS/MS, adverse event