110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

安罗替尼对于接受标准疗法后出现进展的晚期 NSCLC 患者的疗效和安全性以及对疗效预测指标的初步分析
Authors Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S
Received 10 March 2020
Accepted for publication 12 June 2020
Published 12 July 2020 Volume 2020:12 Pages 5641—5650
DOI https://doi.org/10.2147/CMAR.S253366
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Background: The aim of this study was to investigate the efficacy and safety of anlotinib for patients with advanced non-small cell lung cancer (NSCLC) who progressed after standard regimens in real world situations and the preliminary analysis of an efficacy predictor.
Methods: A total of 118 patients with advanced NSCLC who progressed after standard regimens were included in this retrospective study. Efficacy was evaluated and toxicity profile was recorded. Progression-free survival (PFS) and overall survival (OS) were assessed using Kaplan–Meier survival curve and multivariate analysis was adjusted using Cox regression analysis.
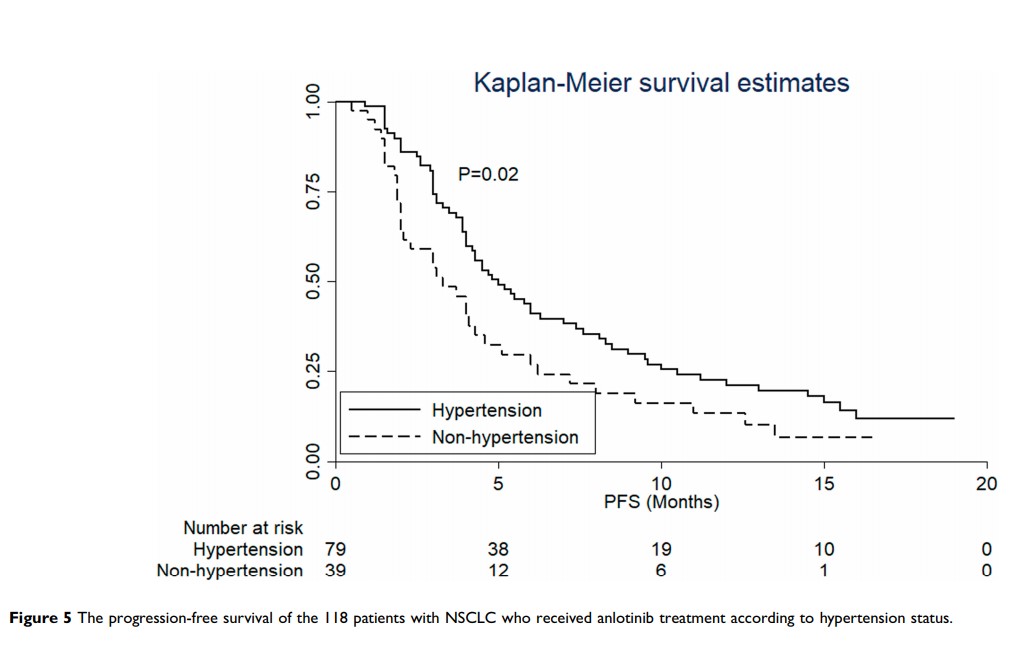
Results: All of the 118 patients with NSCLC were available for evaluation of efficacy. Complete response (CR, 0 case), partial response (PR, 10 cases), stable disease (SD, 79 cases) and progressive disease (PD, 29 cases) were evaluated according to RECIST version 1.1. In consequence, objective response rate (ORR) was 8.47% and disease control rate (DCR) was 75.42%. The median PFS of the 118 patients with NSCLC was 4.3 months and the median OS was 10.3 months. The results of Cox regression analysis suggested that ECOG score was an independent factor for PFS. The toxicity profile indicated that hypertension and hand-foot syndrome were the most common adverse reactions. Additionally, the preliminary analysis of an efficacy predictor suggested that the PFS of patients with hypertension was superior to those without hypertension.
Conclusion: Anlotinib is effective and safe for patients with advanced NSCLC who progressed after standard regimens in real world situations. Hypertension may be a biomarker for efficacy prediction.
Keywords: non-small cell lung cancer, anlotinib, efficacy, safety, biomarker