108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过一种新型 c-Met/CD3 双特异性抗体抑制 c-Met 过表达的肿瘤
Authors Huang L, Xie K, Li H, Wang R, Xu X, Chen K, Gu H, Fang J
Received 18 March 2020
Accepted for publication 17 July 2020
Published 7 August 2020 Volume 2020:14 Pages 3201—3214
DOI https://doi.org/10.2147/DDDT.S254117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Introduction: Overexpression of c-Met, or hepatocyte growth factor (HGF) receptor, is commonly observed in tumor biopsies and often associated with poor patient survival, which makes HGF/c-Met pathway an attractive molecular target for cancer therapy. A number of antibody-based therapeutic strategies have been explored to block c-Met or HGF in cancers; however, clinical efficacy has been very limited, indicating that blockade of c-Met signal alone is not sufficient. Thus, an alternative approach is to develop an immunotherapy strategy for c-Met-overexpressing cancers. c-Met/CD3 bispecific antibody (BsAb) could bridge CD3-positive T lymphocytes and tumor cells to result in potent tumor cell killing.
Materials and Methods: A bispecific antibody, BS001, which binds both c-Met and CD3, was generated using a novel BsAb platform. Western blotting and T cells-mediated killing assays were utilized to evaluate the BsAb’s effects on cell proliferation, survival and signal transduction in tumor cells. Subcutaneous tumor mouse models were used to analyze the in vivo anti-tumor effects of the bispecific antibody and its combination therapy with PD-L1 antibody.
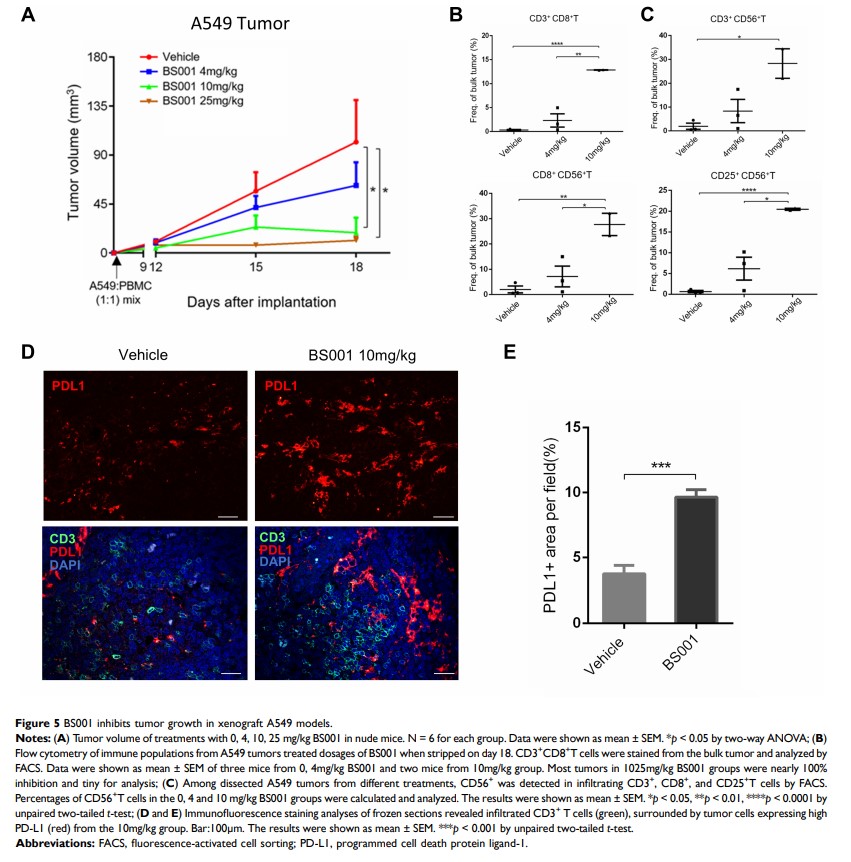
Results: BS001 showed potent T-cell mediated tumor cells killing in vitro. Furthermore, BS001 inhibited phosphorylation of c-Met and downstream signal transduction in tumor cells. In A549 lung cancer xenograft model, BS001 inhibited tumor growth and increased the proportion of activated CD56+ tumor infiltrating lymphocytes. In vivo combination therapy of BS001 with Atezolizumab (an anti-programmed cell death protein1-ligand (PD-L1) antibody) showed more potent tumor inhibition than monotherapies. Similarly, in SKOV3 xenograft model, BS001 showed a significant efficacy in tumor growth inhibition and tumor recurrence was not observed in more than half of mice treated with a combination of BS001 and Pembrolizumab.
Conclusion: c-Met/CD3 bispecific antibody BS001 exhibited potent anti-tumor activities in vitro and in vivo, which was achieved through two distinguished mechanisms: through antibody-mediated tumor cell killing by T cells and through inhibition of c-Met signal transduction.
Keywords: c-Met, bispecific antibody, lung cancer, ovarian cancer, checkpoint antibody