108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

0.01% 阿托品滴眼液用于治疗近视眼时对儿童眼表的影响 – 一项为期六个月的前瞻性研究的主要结果
Authors Cheng J, Yang Y, Kong X, Zeng L, Chen Z, Xu J, Zhang C
Received 8 June 2020
Accepted for publication 27 July 2020
Published 10 August 2020 Volume 2020:16 Pages 735—740
DOI https://doi.org/10.2147/TCRM.S265945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Purpose: To evaluate the effect of 0.01% atropine eye drops on the ocular surface in children for the control of myopia.
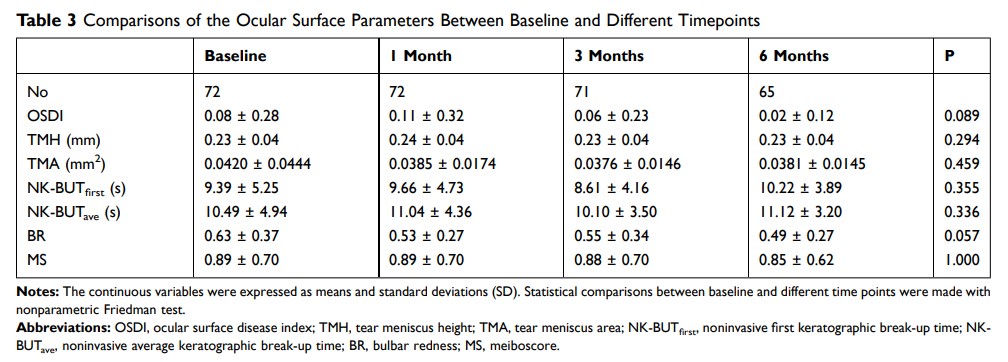
Methods: A total of 72 participants were recruited for this prospective study. Prior to and after 1, 3, and 6 months of 0.01% atropine administration, an ocular surface disease index (OSDI) questionnaire was obtained, Keratograph 5M was used for the measurement of the tear meniscus height (TMH), noninvasive keratographic tear film break-up time (NK-BUT, the first keratographic break-up time, [NK-BUTfirst] and the average keratographic break-up time, [NK-BUTave]), bulbar redness (BR), meiboscore (MS), and anterior segment optical coherence tomography (AS-OCT) was used to calculate the inferior tear meniscus area (TMA).
Results: After using the 0.01% atropine eye drops for 1 month, 9 subjects complained of discomfort immediately after administration, but this quickly subsided, and 1 subject was temporarily dazzled. All the ocular surface symptoms were mild and occurred rarely. After 3 months, these complaints no longer occurred. Compared with the baseline values, the OSDI scores (0.08 ± 0.28), values of TMH (0.23 ± 0.04 mm), TMA (0.0420 ± 0.0444 mm2), NK-BUTfirst (9.39 ± 5.25 s), NK-BUTave (10.49 ± 4.94 s), BR (0.63 ± 0.37), and MS (0.89 ± 0.70) did not change significantly after 6 months of 0.01% atropine eye drop administration (P > 0.05).
Conclusion: In this 6-month prospective study, no side effects were observed on the ocular surface after using 0.01% atropine in children.
Keywords: 0.01% atropine eye drops, ocular surface, children, myopia control