108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

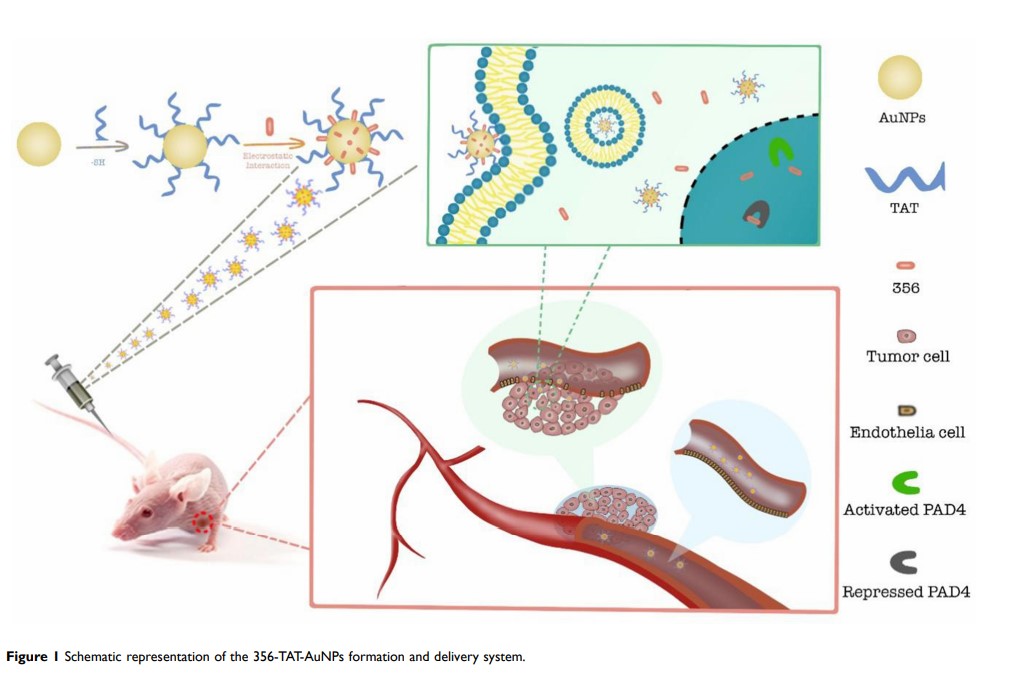
TAT 修饰的金纳米颗粒增强 PAD4 抑制剂的抗肿瘤活性
Authors Song S, Gui L, Feng Q, Taledaohan A, Li Y, Wang W, Wang Y, Wang Y
Received 2 April 2020
Accepted for publication 5 August 2020
Published 10 September 2020 Volume 2020:15 Pages 6659—6671
DOI https://doi.org/10.2147/IJN.S255546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Purpose: Histone citrullination by peptidylarginine deiminases 4 (PAD4) regulates the gene expression of tumor suppressor. In our previously study, YW3-56 (356) was developed as a potent PAD4 inhibitor for cancer therapy with novel function in the autophagy pathway. To enhance the antitumor activity, the PAD4 inhibitor 356 was modified by the well-established cationic penetrating peptide RKKRRQRRR (peptide TAT) and gold nanoparticles to obtain 356-TAT-AuNPs which could enhance the permeability of chemical drug in solid tumor.
Methods: 356-TAT-AuNPs were prepared, and their morphology were characterized. The antitumor activity of 356-TAT-AuNPs was evaluated in vitro and in vivo.
Results: 356-TAT-AuNPs exhibited higher anticancer activity against HCT-116, MCF-7 and A549 cells than 356 and 356-AuNPs. Compared with 356 and 356-AuNPs, 356-TAT-AuNPs entered the cytoplasm and nuclear, exhibited stronger anticancer activity by increasing apoptosis, inducing autophagy and inhibiting of histone H3 citrullination, and in HCT-116 xenograft mouse model, 356-TAT-AuNPs could improve the antitumor activity.
Conclusion: The modified AuNPs with peptide TAT as drug delivery system are potent in delaying tumor growth and could be a powerful vehicle for profitable anticancer drug development. We believe that peptide TAT modification strategy may provide a simple and valuable method for improving antitumor activity of PAD4 inhibitors for clinical use.
Keywords: Au nanoparticle, transmembrane peptide, PAD4 inhibitor, antitumor