108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

局部晚期胃癌新辅助化疗的回顾性研究
Authors Wang Y, He K, Zhou Z, Zhong Y, Li G, Lu J
Received 22 June 2020
Accepted for publication 21 August 2020
Published 15 September 2020 Volume 2020:12 Pages 8491—8496
DOI https://doi.org/10.2147/CMAR.S267330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Objective: To explore the efficacy and safety of neoadjuvant chemotherapy in the doublet and triplet regimens of locally advanced gastric cancer.
Patients and Methods: A retrospective analysis was conducted on 162 patients with gastric cancer who received neoadjuvant chemotherapy, including 74 patients receiving doublet regimen (fluorouracil/platinum) and 88 patients receiving triplet regimen (fluorouracil/platinum/Taxol). Patients in both groups received neoadjuvant chemotherapy for two cycles, and underwent surgical resection 4 weeks after the end of chemotherapy.
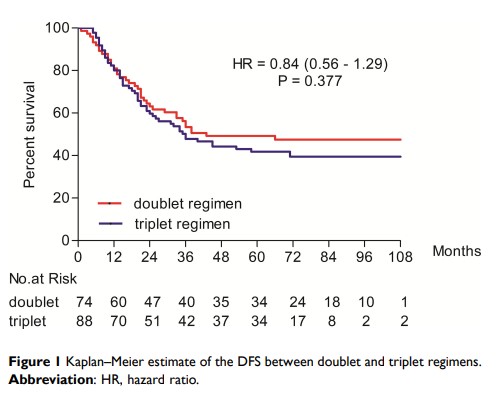
Results: The total clinical remission rate was 68.6% (105/153), the phase-down rate was 46.4% (71/153), and the pathological response rate was 59.9% (97/162). In the doublet and triplet regimen, the clinical remission rate was 65.7% (44/67) and 70.9% (61/86) (P = 0.708), the descending period rate was 41.8% (28/67) and 50.0% (43/86) (P = 0.485), and the pathological response rate was 51.4% (38/74) and 67.0% (59/88) (P = 0.190). The median disease-free survival (DFS) and overall survival (OS) of 162 patients were 36.0 and 58.5 months. In the doublet and triplet regimen, the median DFS was 38.0 and 34.0 months (P = 0.377), and the median OS was 59.0 and 56.5 months (P = 0.256). The side effects of the doublet group were significantly lower than those of the triplet group, with leucopenia rate of 45.9% (34/74) and 62.5% (55/88) (P = 0.035); thrombocytopenia rate of 18.9% (14/74) and 35.2% (31/88) (P = 0.021); nausea rate of 45.9% (34/74) and 64.8% (57/88) (P = 0.016), and diarrhea rate of 1.4% (1/74) and 9.1% (8/88) (P = 0.032).
Conclusion: Neoadjuvant chemotherapy is safe and effective for locally advanced gastric cancer. The clinical efficacy of neoadjuvant chemotherapy in the doublet group and the triplet group is equivalent, and the doublet group has better safety and tolerance.
Keywords: locally advanced gastric cancer, neoadjuvant chemotherapy, doublet regimen, triplet regimen, prognosis