108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

享受呼吸计划:一个改善中国初级卫生保健系统慢性阻塞性肺疾病管理的全国前瞻性研究方案
Authors Jia C, Zhang C, Fang F, Huang K, Dong F, Gu X, Niu H, Li S, Wang C, Yang T
Received 16 April 2020
Accepted for publication 15 July 2020
Published 15 September 2020 Volume 2020:15 Pages 2179—2187
DOI https://doi.org/10.2147/COPD.S258479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
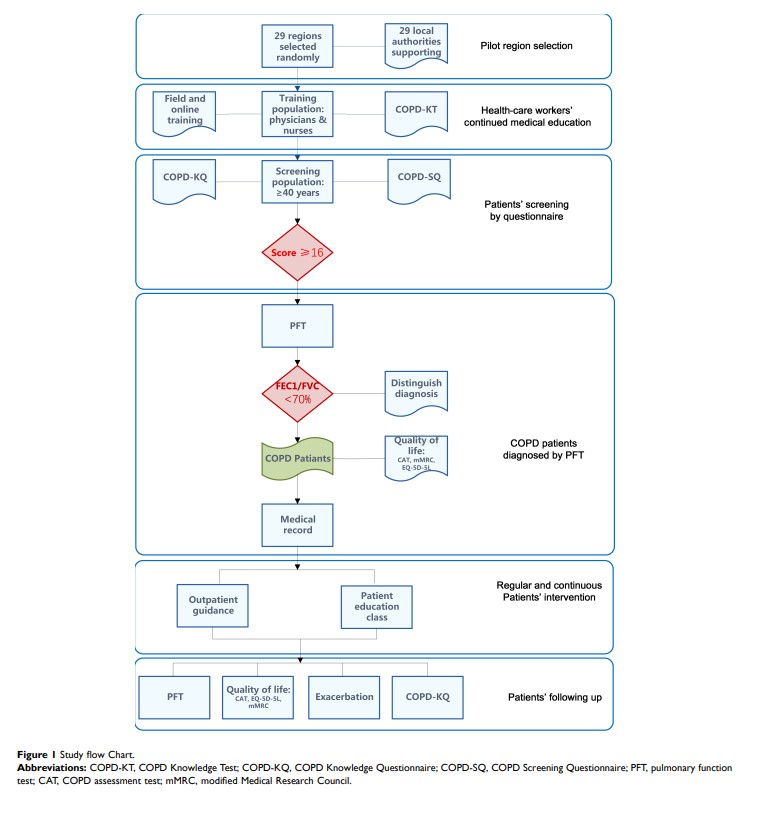
Background: Chronic obstructive pulmonary disease (COPD) is prevalent and poses a heavy burden worldwide. However, patients know little about COPD, and primary health care providers have poor therapy capability in China. Enjoying Breathing Program aims to establish a new comprehensive COPD patient management system, including early detection, standardized therapy, and follow-up in China. The goal of the study is to 1) describe the intervention for physicians and patients and 2) to assess the effectiveness of this program.
Methods: It is the first nationwide trial involving all levels of health care institutions from primary health care institutions to tertiary hospitals. It includes a series of structured but individualized intervention for both health care providers and COPD patients. Primary health care providers from pilot hospitals will take both online and face-to-face courses, including the procedure of COPD patients’ management and prevention, diagnosis and treatment. Once the patients are diagnosed with COPD, they will undertake standard therapy and self-management education program, perform rehabilitation exercises, and be followed up by primary health care providers every 3 months. The primary outcome will be exacerbation-related hospital/emergency admission and the change of patients’ awareness and primary health care providers’ knowledge of COPD within 36 months. Secondary outcome will include the change of pulmonary function test, structured COPD patients’ management, two-way referral, and standardized therapy.
Conclusion: A comprehensive COPD patient management model to promote the standardized therapy will be established; this will improve COPD patients’ awareness and health quality.
Trial Registration Number: This study has been registered at www.ClinicalTrials.gov (registration identifier: NCT04318912).
Keywords: protocol, national prospective trial, COPD management