108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

高良姜素(GLN)通过靶向 Skp2 诱导上皮-间质转化(EMT),从而抑制人胶质母细胞瘤细胞的增殖、迁移和侵袭
Authors Xiong Y, Lai X, Xiang W, Zhou J, Han J, Li H, Deng H, Liu L, Peng J, Chen L
Received 28 May 2020
Accepted for publication 29 July 2020
Published 17 September 2020 Volume 2020:13 Pages 9235—9244
DOI https://doi.org/10.2147/OTT.S264209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
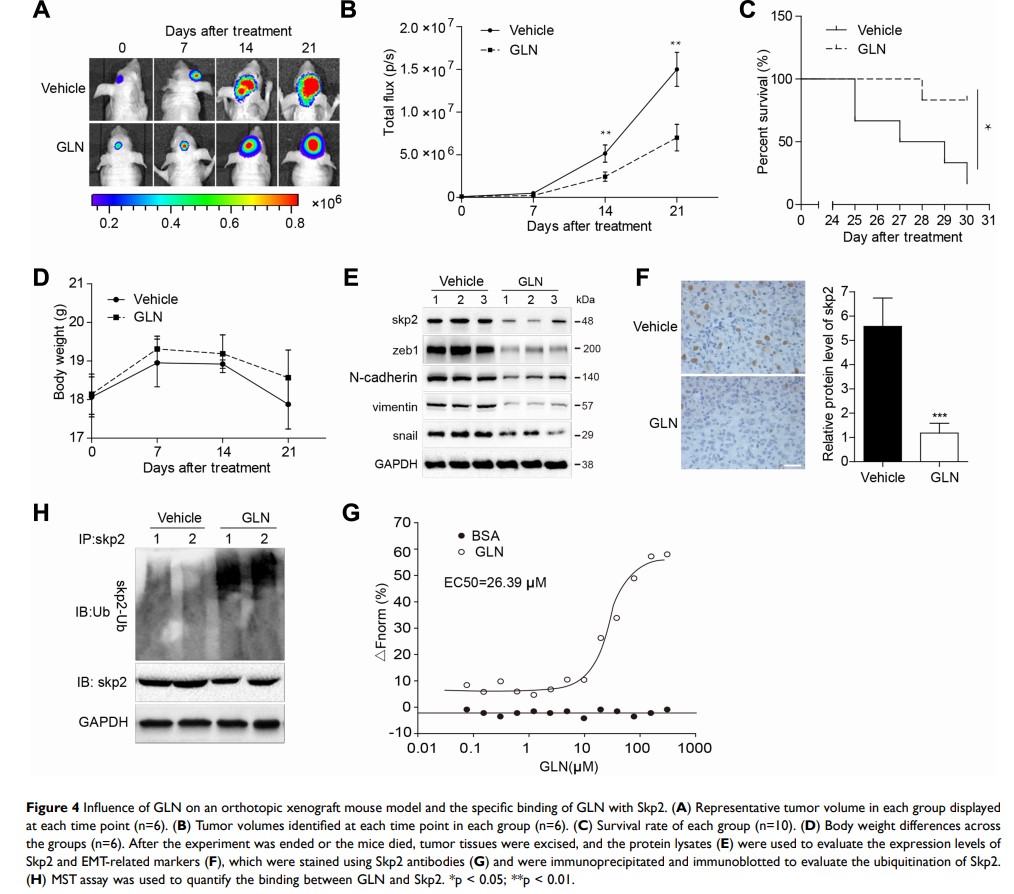
Background: Galangin (GLN), a pure natural flavonoid compound found in plants, has been shown to exert anti-cancer effects against multiple cancer types, including glioma. However, its underlying molecular mechanism remains unclear. Epithelial-to-mesenchymal transition (EMT) performs an important function in the genesis and development of cancer. Skp2, a pivotal component of SCFSkp2 E3 ubiquitin ligase, has been shown to function as an oncogene in GBM invasion that contributes to the EMT process. Thus, we explored whether GLN inhibited Skp2-mediated EMT and the mechanism underlying the Skp2 degradation pathway.
Methods: CCK-8 assay, wound healing assay and transwell assay were used to examine cell proliferation, migration, and invasion after treatment with or without GLN. RT-PCR and Western blotting analysis were performed to evaluate mRNA and protein expression, respectively. Co-immunoprecipitation was conducted to detect ubiquitinated Skp2 levels in vitro and in vivo after GLN treatment. Bioluminescence imaging was performed to examine the intracranial tumor size of U87 xenograft mice. Microscale thermophoresis (MST) experiment was used to detect interactions between Skp2 and GLN.
Results: GLN suppressed GBM cell growth, migration, and invasion, and also downregulated the expression of Skp2 and mesenchymal markers (Zeb1, N-cadherin, snail, vimentin) in vitro. Moreover, the overexpression of Skp2 in GBM cells decreased the effect of GLN on EMT. Furthermore, we demonstrated that GLN degraded skp2 protein through the ubiquitination proteasome pathway and directly interacted with skp2 protein, as shown through the MST assay.
Conclusion: This study is the first to identify Skp2 as a novel target of GLN for the treatment of GBM and report of Skp2 protein degradation in a ubiquitination proteasome pathway. Results from our study indicated the potential of GLN for the treatment of GBM through ubiquitin-mediated degradation of Skp2.
Keywords: galangin, GBM, EMT, Skp2, ubiquitination