108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

确定载脂蛋白 E 为鼻咽癌的潜在诊断生物标志物
Authors Xue Y, Huang S, Huang J, Li S, Zhang C, Zhou X
Received 21 November 2019
Accepted for publication 20 July 2020
Published 24 September 2020 Volume 2020:12 Pages 8943—8950
DOI https://doi.org/10.2147/CMAR.S239479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev Srivastava
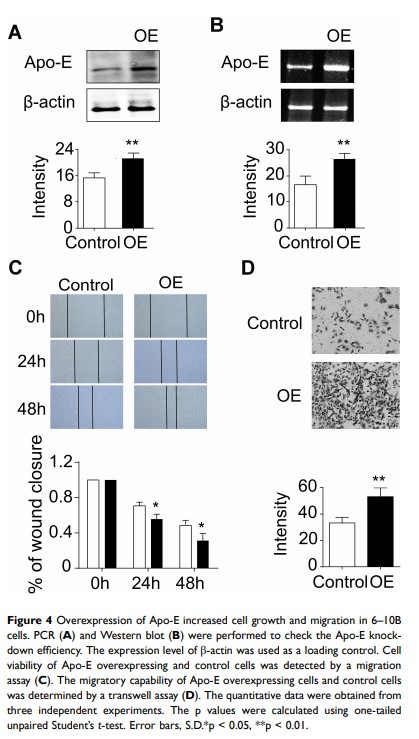
Purpose: Apo-E, a secreted protein, is closely related to the migration and invasion of tumor cells. In this study, we aimed to analyze the expression of Apo-E in nasopharyngeal carcinoma (NPC) patients and cell lines, as well as its effects on NPC cell behavior.
Patients and Methods: Our study included 35 patients with NPC from Zhongnan Hospital. Expression levels of Apo-E in patients with NPC were examined by quantitative reverse transcription-polymerase chain reaction, Western blot, and immunohistochemical (IHC) staining. Receiver operating characteristic (ROC) curves were analyzed using the SPSS 22 software to estimate the sensitivity and specificity of the Apo-E protein in diagnosing NPC. Additionally, the level of Apo-E in NPC cell lines (NP69, 6– 10B, and 5– 8F) was investigated by Western blotting and IHC.
Results: Levels of Apo-E were higher in NPC patients than in controls. Moreover, ROC analysis revealed that increased Apo-E in the serum of NPC patients may act as a potential biomarker for NPC diagnosis (Area under the curve 0.917). Furthermore, similar results were also identified in NPC cancer cell lines. RNA interference technology was used to overexpress the endogenous Apo-E in the NPC cell line 6– 10B. Wound healing and transwell assays indicated that the overexpression of Apo-E increased the number of cell colonies and migration ability, respectively.
Conclusion: In this study, we found that Apo-E was elevated in NPC patients and may act as a potential biomarker for NPC diagnosis. In addition, Apo-E was upregulated in NPC cell lines and promoted cell growth, migration, and invasion.
Keywords: cancer, nasopharynx, secretory glycoprotein, migration, invasion