108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

使用间充质干细胞治疗 COVID-19 的挑战
Authors Li C, Zhao H, Wang B
Received 25 June 2020
Accepted for publication 6 September 2020
Published 29 September 2020 Volume 2020:14 Pages 3995—4001
DOI https://doi.org/10.2147/DDDT.S269407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Zhu
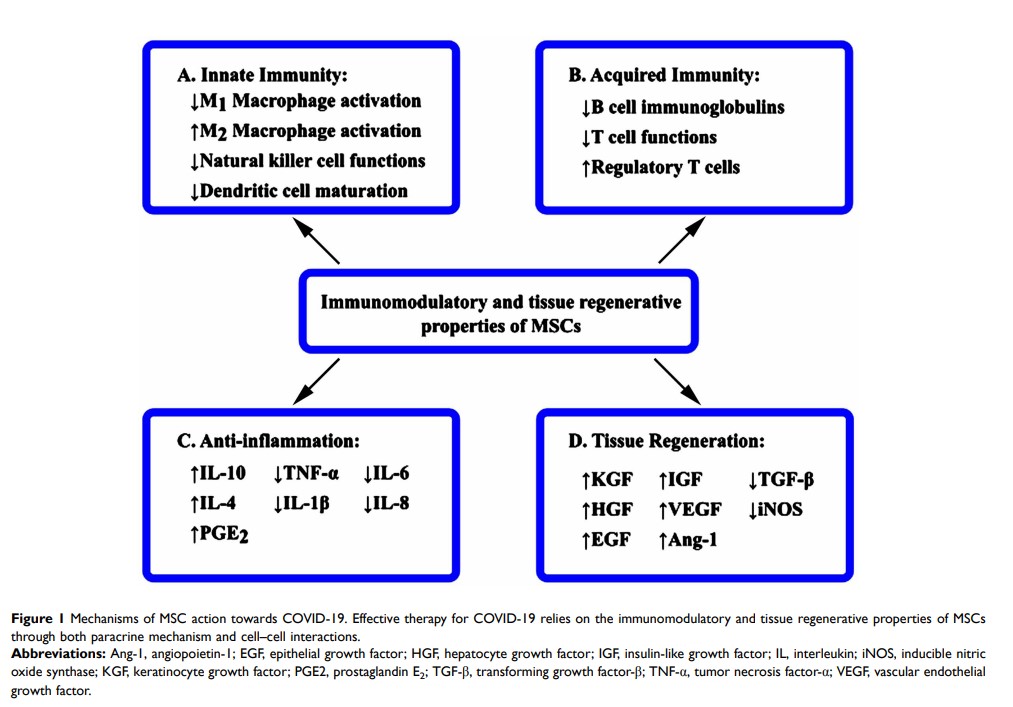
Abstract: The coronavirus disease 2019 (COVID-19) global pandemic continues and antiviral agents and vaccines are currently under investigation. Mesenchymal stem cell (MSC)-based therapy can be a suitable option for management of patients with COVID-19 at the urgent time of virus outbreak. Currently, MSCs are being explored against the novel infectious disease due to their therapeutic properties of anti-inflammation, immunomodulation and tissue repair and regeneration, albeit the precise mechanisms of MSC action toward COVID-19 remain unclear. To date, rigorous results from clinical trials using MSCs in human have been weakly positive. The pervasive uncertainty of using MSC therapeutic products as an effective combatant against COVID-19 requires rigorous resolution on several fronts, including MSC fate after infusion, safety issue, homing capability, and MSC resistance to the disease microenvironment. Focusing on these facets, a few important ones will be critically analyzed and addressed in this article for the development of safe and effective MSC-based therapies for COVID-19.
Keywords: COVID-19, mesenchymal stem cell, immunomodulation, tissue regeneration