108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

使用载有多孔微晶胶介质的脂肪源性间充质干细胞构建微单元,以修复大鼠模型的急性跟腱断裂
Authors Yang X, Meng H, Peng J, Xu L, Wang Y, Sun X, Zhao Y, Quan Q, Yu W, Chen M, Shi T, Du Y, Lu S, Wang A
Received 13 November 2019
Accepted for publication 28 August 2020
Published 29 September 2020 Volume 2020:15 Pages 7155—7171
DOI https://doi.org/10.2147/IJN.S238399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
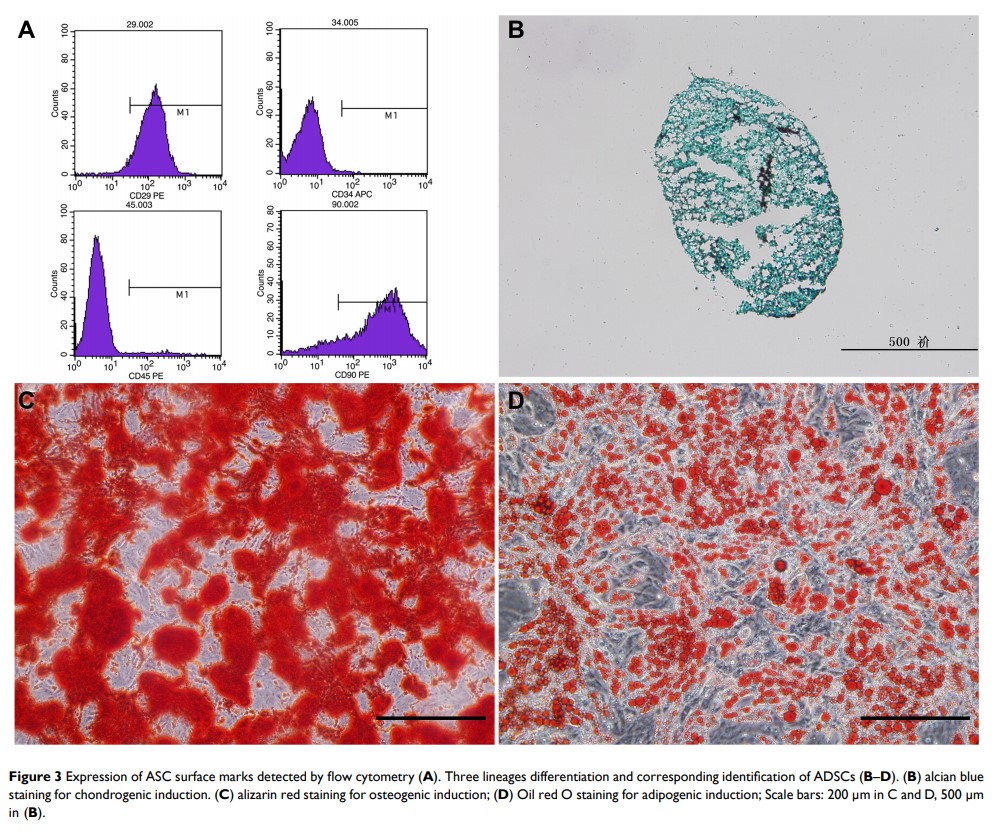
Objective: Tissue engineering approaches seem to be an attractive therapy for tendon rupture. Novel injectable porous gelatin microcryogels (GMs) can promote cell attachment and proliferation, thus facilitating the repair potential for target tissue regeneration. The research objectives of this study were to assess the efficacy of tissue-like microunits constructed by multiple GMs laden with adipose-derived mesenchymal stem cells (ASCs) in accelerated tendon regeneration in a rat model.
Methods: Through a series of experiments, such as isolation and identification of ASCs, scanning electron microscopy, mercury intrusion porosimetry (MIP), laser scanning confocal microscopy and the CCK-8 test, the biocompatibility of GMs was evaluated. In an in vivo study, 64 rat right transected Achilles tendons were randomly divided into four groups: the ASCs+GMs group (microunits aggregated by multiple ASC-laden GMs injected into the gap), the ASCs group (ASCs injected into the gap), the GMs group (GMs injected into the gap) and the blank defect group (non-treated). At 2 and 4 weeks postoperatively, the healing tissue was harvested to evaluate the gross observation and scoring, biomechanical testing, histological staining and quantitative scoring. Gait analysis was performed over time. The 64 rats were randomly assigned into 4 groups: (1) micro-unit group (ASCs+GMs) containing ASC (105)-loaded 120 GMs in 60 μL DMEM; (2) cell control group (ASCs) containing 106 ASCs in 60 μL DMEM; (3) GM control group (GMs) containing 120 blank GMs in 60 μL DMEM; (4) blank defect group (Defect) containing 60 μL DMEM, which were injected into the defect sites. All animals were sacrificed at 2 and 4 weeks postsurgery (Table 1).
Results: In an in vitro study, GMs (from 126 μm to 348 μm) showed good porosities and a three-dimensional void structure with a good interpore connectivity of the micropores and exhibited excellent biocompatibility with ASCs. As the culture time elapsed, the extracellular matrix (ECM) secreted by ASCs encased the GMs, bound multiple microspheres together, and then formed active tendon tissue-engineering microunits. In animal experiments, the ASCs+GMs group and the ASCs group showed stimulatory effects on Achilles tendon healing. Moreover, the ASCs+GMs group was the best at improving the macroscopic appearance, histological morphology, Achilles functional index (AFI), and biomechanical properties of repair tissue without causing adverse immune reactions.
Conclusion: Porous GMs were conducive to promoting cell proliferation and facilitating ECM secretion. The ASCs-GMs matrices showed an obvious therapeutic efficiency for Achilles tendon rupture in rats.
Keywords: Achilles tendon rupture, tendon tissue engineering, adipose-derived mesenchymal stem cells, ASCs, injectable biomaterials