108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

安洛替尼用于铂类耐药卵巢癌的探索性疗法:疗效和安全性的回顾性研究
Authors Ni J, Cheng X, Chen J, Guo W, Dai Z
Received 20 June 2020
Accepted for publication 12 August 2020
Published 5 October 2020 Volume 2020:13 Pages 9857—9863
DOI https://doi.org/10.2147/OTT.S268613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Purpose: Survival of platinum-resistant ovarian cancer (PROC) patients is significantly shortened to around 12 months. Anlotinib is a new multi-target tyrosine kinase inhibitor. The goal of this study is to evaluate the efficacy and safety of anlotinib in PROC patients.
Patients and Methods: PROC patients treated with anlotinib in Jiangsu Cancer Hospital between June 2018 to September 2019 were recruited. Most patients received an initial bolus of 12mg orally once daily on days 1– 14 of a 21-day cycle (except one received a dose of 10mg and another one received a dose of 8mg orally once a day). The adverse events (AEs) and efficacy were analyzed by CTCAE 4.0 and RECIST 1.1.
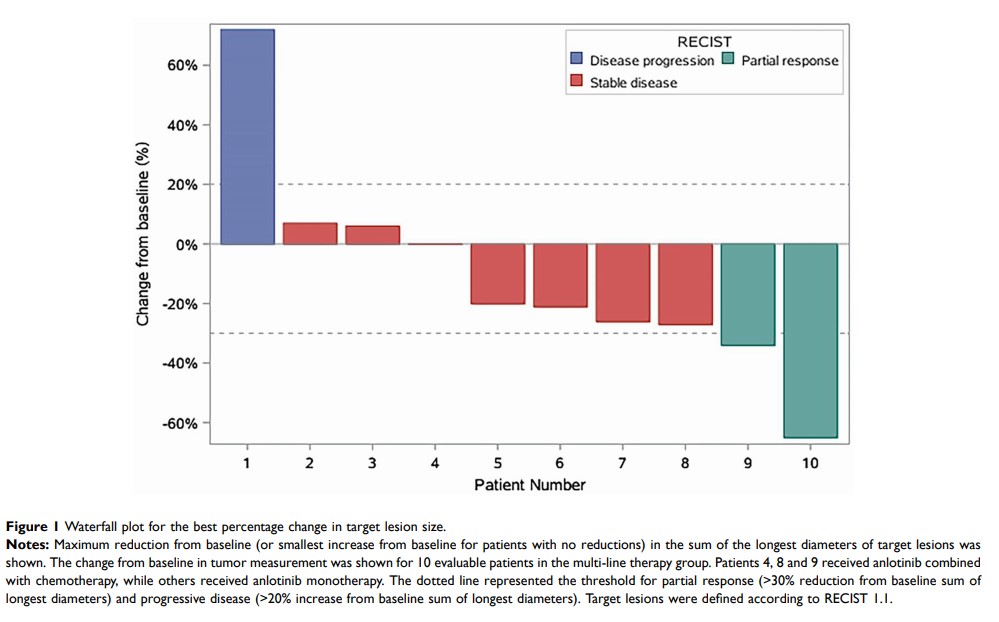
Results: Of all 15 enrolled patients, 12 patients received anlotinib as multi-line therapy and 3 patients received it as maintenance therapy. In the multi-line therapy group, eight patients received anlotinib monotherapy and four patients received anlotinib combined with chemotherapy. Ultimately, evaluation showed that one patient achieved partial response (PR), five patients achieved stable disease (SD) and one patient had progressive disease (PD) with monotherapy, yielding objective response rate (ORR) of 14.3% (95% CI=0.01– 0.58) and disease control rate (DCR) of 85.7% (95% CI=0.42– 0.99). One patient achieved PR, two patients achieved SD with combination therapy, yielding ORR of 33.3% (95% CI=0.02– 0.87) and DCR of 100% (95% CI=0.31– 1.00). Three patients with maintenance therapy were followed up for 5, 8, and 11 months, respectively. The most grade 1– 2 AEs were hand-foot syndrome, nausea, and hypertension. Serious AEs (SAEs) (Grade 3– 4) were observed in one patient with oral ulcer and another patient with hand-foot syndrome that were managed by dose reduction.
Conclusion: Anlotinib was of promising efficacy and well tolerated in PROC patients. This is the first retrospective study about exploratory therapy for ovarian cancer patients with anlotinib.
Keywords: platinum-resistant ovarian cancer, anlotinib, efficacy, safety