110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape
Authors Subramanian J, Cavenagh J, Desai B, Jacobs I
Received 25 August 2016
Accepted for publication 5 January 2017
Published 24 April 2017 Volume 2017:9 Pages 131—140
DOI https://doi.org/10.2147/CMAR.S120589
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Kexin Xu
Abstract: Follicular lymphoma (FL) is the second most common type of non-Hodgkin’s
lymphoma. FL is an incurable disease with treatment options ranging from a
“watch-and-wait” approach to localized therapy with radiation or systemic
therapy with rituximab in combination with chemotherapy regimens. This review
summarizes the role of rituximab across the spectrum of FL treatment and the
evolving therapeutic landscape with the emergence of novel agents currently in
clinical development. Despite the prospect of new agents on the horizon, it is
widely accepted that rituximab will remain as the cornerstone of therapy
because of its established long-term efficacy. Many biologics, including
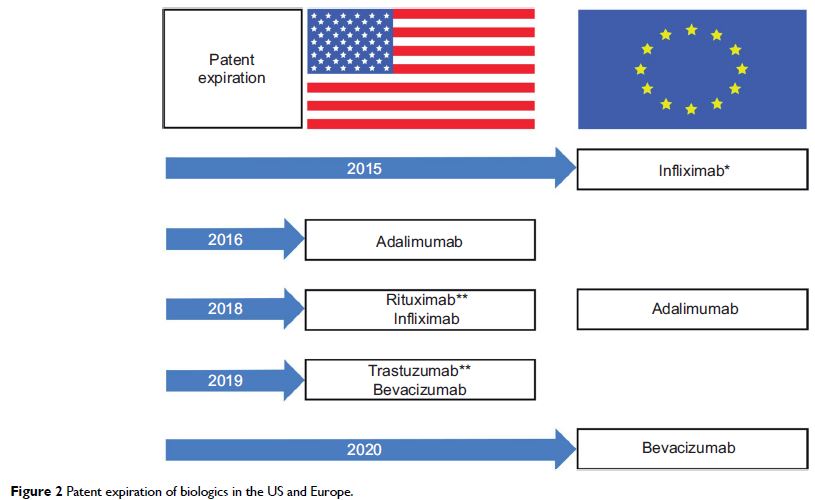
rituximab, have lost exclusivity of composition-of-matter patent or will do so
in the next few years, which is a concern for patients and physicians alike.
Moreover, access to rituximab is challenging, particularly in countries with
restricted resources. Together, these concerns have fueled the development of
safe and effective biosimilars. The term “biosimilar” refers to a biologic
product that is highly similar to an approved reference (or originator)
product, notwithstanding minor differences in clinically inactive components,
and for which there are no clinically meaningful differences in purity,
potency, or safety. Biosimilars are developed to treat the same condition(s)
using the same treatment regimens as an approved reference biologic, and have
the potential to increase access to more affordable treatment of FL. Herein, we
also discuss the potential benefits of eagerly awaited rituximab biosimilars,
which may mitigate the impact of the lack of access to rituximab.
Keywords: biosimilar, follicular lymphoma,
non-Hodgkin’s lymphoma