110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

分子靶向药物用于晚期胃癌治疗时出现的,与治疗相关的严重和致命的不良事件:一项综合分析
Authors Wang L, Liu Y, Zhou W, Li W
Received 12 April 2016
Accepted for publication 6 July 2016
Published 26 April 2017 Volume 2017:10 Pages 2281—2287
DOI https://doi.org/10.2147/OTT.S110431
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Manfred Beleut
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Aim: To perform a systematic review and meta-analysis of Phase III randomized
controlled trials (RCTs) to determine the incidence and risk of severe adverse
events (AEs) with molecular targeted agents (MTAs) in advanced/metastatic
gastric cancer (GC) patients.
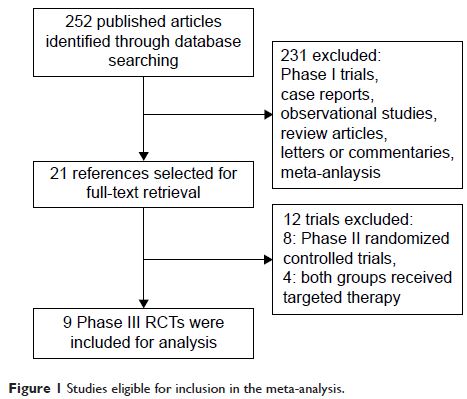
Methods: A comprehensive literature search for related trials
published up to December 2015 was performed. Eligible studies were Phase III
RCTs of advanced/metastatic GC patients assigned to MTAs or control group. Data
were extracted by two authors for severe and fatal AEs (FAEs).
Results: A total of nine Phase III RCTs involved 4,934 GC
patients were ultimately identified. The pooled results demonstrated that the
addition of TAs to therapies in advanced GC significantly increased the risk of
developing severe AEs (relative risk: 1.12, 95% confidence interval: 1.02–1.24, P =0.02), but not
for FAEs (relative risk: 0.97, 95% confidence interval: 0.65–1.45, P =0.88).
Additionally, the most common causes of FAEs with MTAs were infections (16.3%),
gastrointestinal hemorrhage (8.2%), and arterial thromboembolic events (8.2%),
respectively.
Conclusion: With available evidence, the use of TAs in GC patients
was associated with an increased risk of severe AEs, but not for FAE.
Clinicians should be aware of the risk of severe AEs with the administration of
these drugs in these patients.
Keywords: advanced gastric cancer, molecular targeted
agents, randomized, meta-analysis