110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

带内置软件的家庭无创正压通气治疗稳定型高碳酸血症 COPD:一个短期前瞻性、多中心的随机对照试验
Authors Zhou L, Li X, Guan L, Chen J, Guo B, Wu W, Huo Y, Zhou Z, Liang Z, Zhou Y, Tan J, Chen X, Song Y, Chen R
Received 13 November 2016
Accepted for publication 19 February 2017
Published 27 April 2017 Volume 2017:12 Pages 1279—1286
DOI https://doi.org/10.2147/COPD.S127540
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Professor Hsiao-Chi Chuang
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Background: The
benefits of noninvasive positive pressure ventilation (NPPV) in patients with
hypercapnic COPD are controversial. It is presumed that methodology and
appropriate use of NIV ventilator might be crucial for the outcomes. With the
new built-in software, the performance of NIV can be monitored at home, which
can guarantee the compliance and appropriate use. This study investigated
effects of home use of NIV in hypercapnia in COPD patients using the NIV
ventilator with built-in software for monitoring.
Methods: The current multicenter prospective, randomized,
controlled trial enrolled patients with stable GOLD stages III and IV
hypercapnic COPD. Patients were randomly assigned via a computer-generated
randomization sequence, with a block size of four patients, to continue
optimized treatment (control group) or to receive additional NPPV (intervention
group) for 3 months. The primary outcome was arterial carbon dioxide
pressure (PaCO2). Data were derived from
built-in software and analyzed every 4 weeks. Analysis was carried out
with the intention to treat. This study is registered with ClinicalTrials.gov,
number NCT02499718.
Results: Patients were recruited from 20 respiratory units in
China from October 1, 2015, and recruitment was terminated with a record of the
vital statistics on May 31, 2016. A total of 115 patients were randomly
assigned to the NPPV group (n=57) or the control group (n=58). Patients
complied well with NPPV therapy (mean [± standard deviation] day use
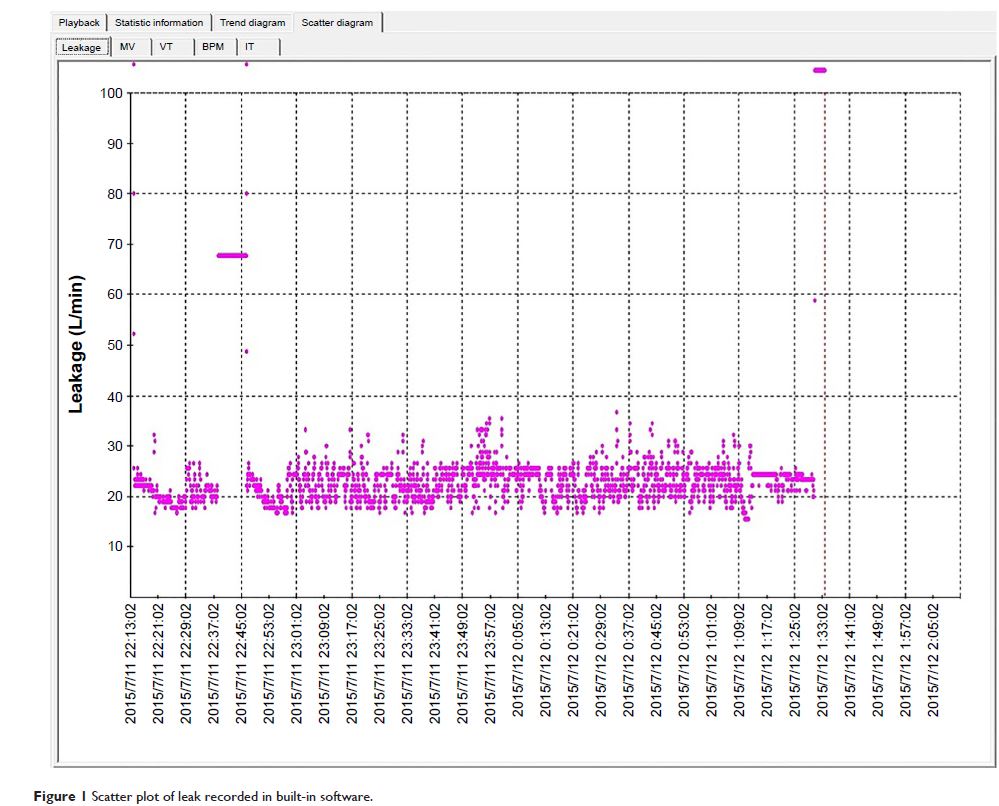
5.6±1.4 h). The mean estimation of leaks was 37.99±13.71 L/min. The
changes in PaCO2 (-10.41±0.97 vs -4.32±0.68 mmHg, P =0.03) and 6-min
walk distance (6MWD) (38.2% vs 18.2%, P =0.02) were
statistically significant in the NPPV group versus the control group. COPD
assessment test (CAT) showed a positive trend (P =0.06)
in favor of the NPPV group. Pulmonary function and dyspnea were not different
between groups.
Conclusion: Ventilators equipped with built-in software provided
methodology for monitoring NIV use at home, which could facilitate the
improvement of compliance and quality control of NIV use. It was shown that
three months use of NIV at home could reduce the PaCO2 and
improve exercise tolerance (6MWD) in chronic hypercapnic COPD patients.
Keywords: COPD,
noninvasive positive pressure ventilation, long-term oxygen therapy, chronic
respiratory failure, built-in software