110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Microbiome disruption and recovery in the fish Gambusia affinis following exposure to broad-spectrum antibiotic
Authors Carlson JM, Leonard AB, Hyde ER, Petrosino JF, Primm TP
Received 30 November 2016
Accepted for publication 28 February 2017
Published 9 May 2017 Volume 2017:10 Pages 143—154
DOI https://doi.org/10.2147/IDR.S129055
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Abstract: Antibiotics are a relatively common disturbance to the normal microbiota
of humans and agricultural animals, sometimes resulting in severe side effects
such as antibiotic-associated enterocolitis. Gambusia affinis was used as a vertebrate model for
effects of a broad-spectrum antibiotic, rifampicin, on the skin and gut mucosal
microbiomes. The fish were exposed to the antibiotic in the water column for 1
week, and then monitored during recovery. As observed via culture, viable
counts from the skin microbiome dropped strongly yet returned to pretreatment
levels by 1.6 days and became >70% resistant. The gut microbiome counts
dropped and took longer to recover (2.6 days), and became >90% drug
resistant. The resistance persisted at ~20% of skin counts in the absence of
antibiotic selection for 2 weeks. A community biochemical analysis measuring
the presence/absence of 31 activities observed a 39% change in results after 3
days of antibiotic treatment. The antibiotic lowered the skin and gut
microbiome community diversity and altered taxonomic composition, observed by
16S rRNA profiling. A 1-week recovery period did not return diversity or
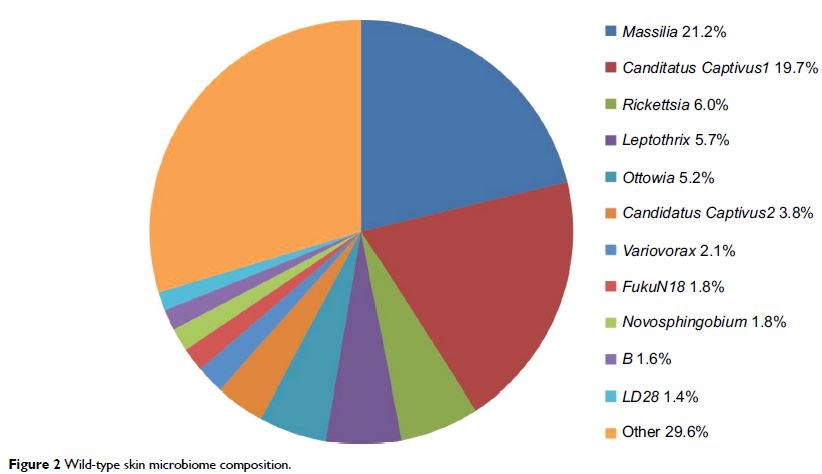
composition to pretreatment levels. The genus Myroides dominated both the microbiomes during
the treatment, but was not stable and declined in abundance over time during
recovery. Rifampicin selected for members of the family Comamonadaceae in the skin
but not the gut microbiome. Consistent with other studies, this tractable
animal model shows lasting effects on mucosal microbiomes following antibiotic
exposure, including persistence of drug-resistant organisms in the community.
Keywords: microbiome, antibiotic, antibiotic
resistance, Gambusia affinis , community
disruption