110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

培美曲塞 (Pemetrexed) 对中国复发性原发中枢神经系统淋巴瘤的疗效和安全性:一项前瞻性研究
Authors Sun Y, Wang Y, Han S, Xing B, Li H, Zhu Y, Zhou S, Wang X, Xu J, Tao R
Received 14 February 2017
Accepted for publication 4 April 2017
Published 17 May 2017 Volume 2017:10 Pages 2595—2600
DOI https://doi.org/10.2147/OTT.S134684
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Carlos Vigil Gonzales
Background: Pemetrexed, a new and novel agent for primary central nervous system
lymphomas (PCNSLs), has shown to be efficient as a savage therapy for recurrent
PCNSLs. However, more studies are needed. A prospective study was performed on
17 recurrent PCNSL patients with pemetrexed at Shandong Tumor Hospital in China
to assess the efficacy and safety of pemetrexed for recurrent PCNSL patients.
Materials and methods: The medical records and imaging data on all the cases
of recurrent PCNSL patients with pemetrexed in our study were collected during
August 2012 and April 2015. Folic acid, B12, and
dexamethasone were used to induce toxicities related to pemetrexed. Patients
were treated with pemetrexed at a dose of 900 mg/m2 intravenously every 3 weeks, and one
cycle consists of 6 weeks.
Results: A total of 17 cases of recurrent PCNSL patients were
enrolled in our study, including 10 males and 7 females with a median age of
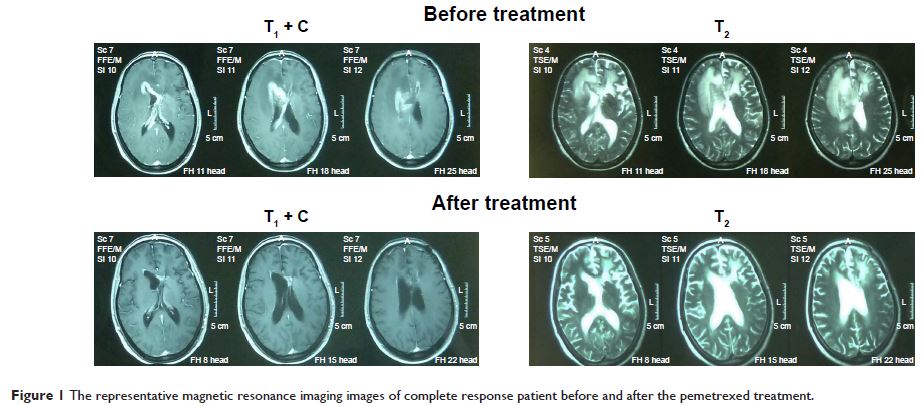
66.2 years (ranging from 35 to 81). After the treatment, five cases had
complete remission, with partial remission in five cases, stable disease
in four cases, and progressive disease in three cases. Consequently, the
overall response rate was 58.8%, and the disease control rate was 82.4%. The median
overall survival was 7.8 months (95% confidence interval: 5.9–9.6 months) in
the study of recurrent PCNSL patients.
Conclusion: This study has been the first clinical trial that
applied pemetrexed to treat recurrent PCNSL patients in China, and results indicated
that chemotherapy using large pemetrexed may become an effective treatment for
PCNSL recurrence with modest toxicity.
Keywords: primary
central nervous system lymphomas, efficacy, safety, recurrence