110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

优化贝伐珠单抗 (Bevacizumab) 治疗,将其作为人表皮生长因子受体 2 (HER2) 阴性晚期乳腺癌的一线治疗:已发表的随机试验的最新综合分析
Authors Li C, Xiang A, Chen X, Yin K, Lu J, Yin W
Received 1 April 2017
Accepted for publication 16 May 2017
Published 27 June 2017 Volume 2017:10 Pages 3155—3168
DOI https://doi.org/10.2147/OTT.S138600
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Lucy Goodman
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Background: Manifold data have demonstrated that the addition of bevacizumab to
chemotherapy improved progression-free survival (PFS), while few trials have
revealed its significant overall survival (OS) benefit. Furthermore, it still
remains suspended how to maximize the benefits of bevacizumab as first-line
therapy for human epidermal growth factor receptor 2 (HER2)-negative breast
cancer. We sought to conduct a meta-analysis to assess the benefits of
bevacizumab with chemotherapy and to identify the ideal chemotherapy partner of
bevacizumab in the first-line setting for HER2-negative advanced breast cancer
patients.
Methods: Computerized and manual searches were performed to identify randomized
clinical trials evaluating the efficacy of bevacizumab plus chemotherapy versus
chemotherapy alone or bevacizumab with different chemotherapy regimens as
first-line therapy for HER2-negative locally recurrent or metastatic breast
cancer patients. Risk ratios or odds ratios with their 95% CIs were used to
estimate the association between multiple combinations of bevacizumab with
chemotherapy and various clinical outcomes.
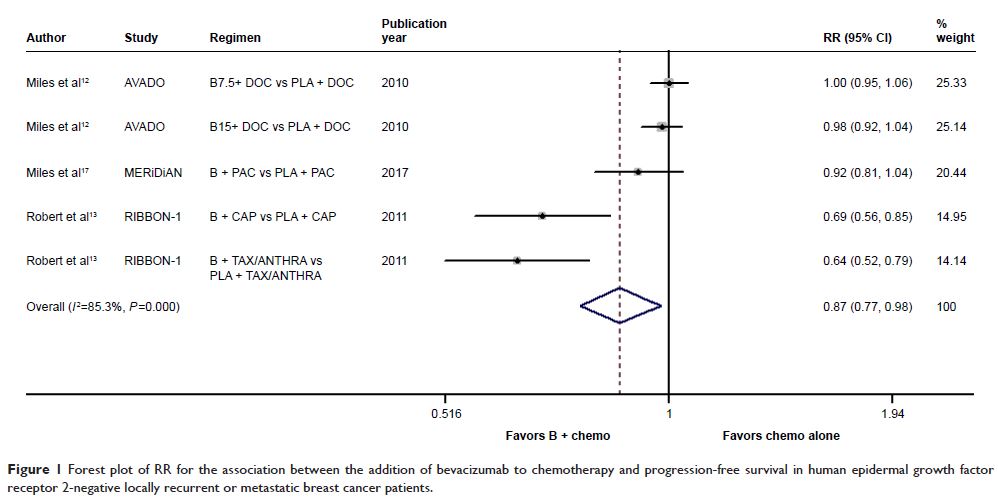
Results: With 7 trials identified, this analysis included 3,984 eligible
patients. The addition of bevacizumab to chemotherapy resulted in a
statistically significant improvement in PFS (P =0.019)
and objective response rate (ORR; P <0.001) rather than in OS (P =0.783) when compared with
chemotherapy alone. The greater benefits in PFS and ORR were achieved from
bevacizumab plus taxane-based regimens compared with bevacizumab plus
capecitabine-based regimens, while bevacizumab plus capecitabine had comparable
OS with bevacizumab plus paclitaxel. Additionally, bevacizumab-based triplet
therapy failed to improve the clinical outcomes when compared with doublet
therapy.
Conclusion: This meta-analysis reveals that the addition of bevacizumab to
chemotherapy yielded PFS and ORR benefits in HER2-negative advanced breast
cancer. Additional studies are still prompted to further optimize the
first-line treatment of bevacizumab.
Keywords: breast cancer, bevacizumab, first-line, HER2-negative, meta-analysis