110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

帕潘立酮棕榈酸酯 (Paliperidone palmitate) 1 个月制剂对中国精神分裂症患者的安全性和有效性: 一个为期 25 周的开放标签、多中心、四期研究
Authors Zhao JP, Li LH, Shi JG, Li Y, Xu XF, Li KQ, Zhang LL, Cai SL, Feng Y, Zhuo JM, Liu WH, Lu HF
Received 29 December 2016
Accepted for publication 11 May 2017
Published 2 August 2017 Volume 2017:13 Pages 2045—2056
DOI https://doi.org/10.2147/NDT.S131224
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Prof. Dr. Roumen Kirov
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Rationale: Long-acting injectable (LAI) paliperidone palmitate 1-month
formulation (PP1M) has demonstrated acceptable tolerability and favorable
clinical outcomes in Western and Asian patients with schizophrenia. Hence,
analysis of the outcomes of long-term PP1M treatment specifically in Chinese
patients is of interest.
Objective: The aim of this study is to evaluate the long-term
safety and efficacy of PP1M treatment in Chinese patients with schizophrenia.
Methods: In this 25-week, open-label, Phase IV study,
patients (18–65 years) diagnosed with schizophrenia and having a baseline
Positive and Negative Syndrome Scale (PANSS) total score of 60–120 (inclusive)
were enrolled. All patients received injections of PP1M 150 mg eq. (day 1)
and 100 mg eq. (day 8), followed by a flexible once-monthly maintenance
dosing (75, 100, or 150 mg eq.).
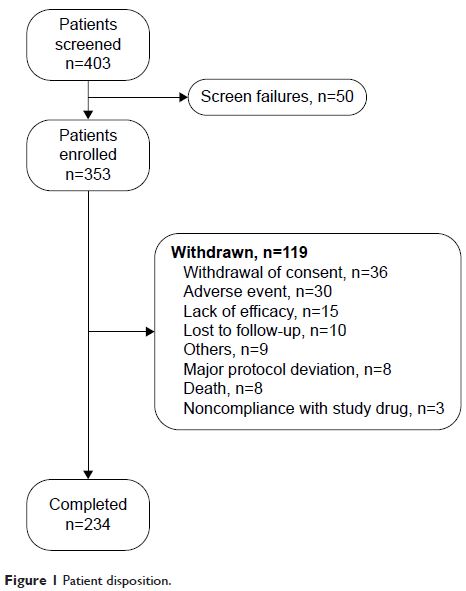
Results: Of the 353 patients, 234 (66.3%) completed the
study treatment (mean age, 31.1 years; 52.7% men). The PANSS total score
(primary end point) improved significantly over the 6-month treatment period
(mean [standard deviation] change from baseline to end of treatment, -27.2
[18.30]; P <0.0001). The Clinical Global
Impressions-Severity and Personal and Social Performance scores (secondary end
points) also improved significantly (P <0.0001). At 6
months, PP1M had a positive impact on medication satisfaction, adherence, and
increased preference for LAIs. Treatment-emergent adverse events (TEAEs) were
reported by 181 (51.3%) patients (TEAEs ≥5%: extrapyramidal disorder [15.3%],
akathisia [10.5%], blood prolactin increase [8.8%], insomnia [5.4%]). A total
of 8 deaths were reported, including 4 completed suicides.
Conclusion: Long-term treatment with PP1M was efficacious,
and no new safety concerns were identified in Chinese patients with
schizophrenia. Overall, the results were comparable with observations from previous
studies.
Keywords: Chinese,
long-acting injectables, open-label, paliperidone palmitate, PANSS,
schizophrenia