110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

致癌性的 miRNAs-130 (OncomiR) 可抑制胃癌中 CRMP4 表达
Authors Zhou Y, Li R, Yu H, Wang R, Shen Z
Received 12 April 2017
Accepted for publication 26 June 2017
Published 3 August 2017 Volume 2017:10 Pages 3893—3905
DOI https://doi.org/10.2147/OTT.S139443
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Abstract: Gastric cancer is one of the most common causes of death
worldwide, although its incidence has steadily declined in recent years. There
is strong evidence that aberrantly expressed microRNAs (miRNAs) are involved in
gastric cancer tumorigenesis. Furthermore, CRMP4 is
closely associated with the occurrence and development of gastric cancer, and
our predictions suggest that miR-130a, which can promote gastric cancer
tumorigenesis, is a potential CRMP4 regulator.
In this study, we investigated the expression of CRMP4 and miR-130a in human
gastric cancer cell lines by quantitative reverse transcription polymerase
chain reaction (qRT-PCR) and Western blot (WB) examination and direct
interactions between miR-130a and CRMP4 by
dual-luciferase reporter assay. We also evaluated the biological roles of
miR-130a and CRMP4 in gastric cancer cells
by flow cytometry, MTT assay, soft agar colony formation assay, and Transwell
tests and confirmed CRMP4 function
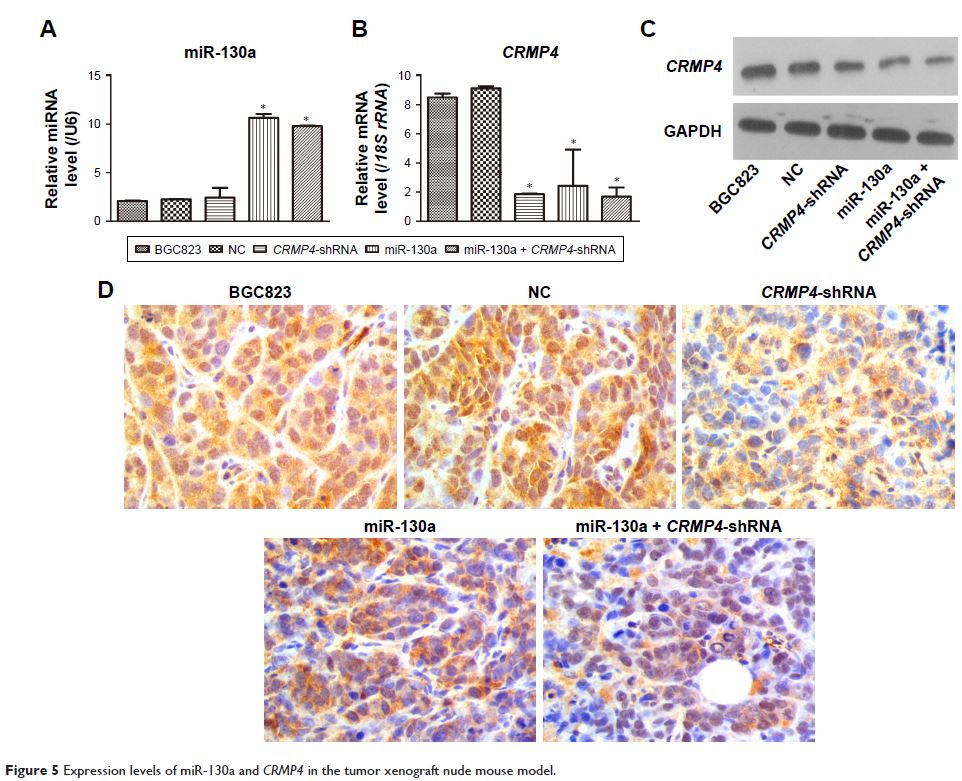
in vivo, using a tumor xenograft model. Our results demonstrated
that CRMP4 expression was
significantly decreased at both the gene and protein levels, while miR-130a
expression was notably increased, in five human gastric cancer cell lines
compared with human gastric epithelial cells. Dual-luciferase reporter assays
indicated that CRMP4 was the
direct target of miR-130a. Moreover, an inverse regulatory relationship between
miR-130a and CRMP4 was verified by qRT-PCR
and WB, and overexpression of miR-130a in BGC823 cells enhanced apoptosis and
cell proliferation, arrested the cell cycle in G0/G1, and facilitated cell
colony formation, invasion, migration, and adhesion, while upregulation
of CRMP4 had opposite effects.
Finally, the growth and weight of transplanted tumors derived from BGC823 cells
in which CRMP4 was knocked down were
remarkably reduced. These data indicate that miR-130a is an oncomir
targeting CRMP4 and could be developed
as a potential prognostic factor and a novel therapeutic target in gastric
cancer.
Keywords: gastric cancer,
microRNA-130a, miR-130a, CRMP4