111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFα-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis
Authors Margaroni M, Agallou M, Athanasiou E, Kammona O, Kiparissides C, Gaitanaki C, Karagouni E
Received 4 May 2017
Accepted for publication 20 June 2017
Published 23 August 2017 Volume 2017:12 Pages 6169—6184
DOI https://doi.org/10.2147/IJN.S141069
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Thiruganesh Ramasamy
Peer reviewer comments 3
Editor who approved publication: Dr Thomas J Webster
Abstract: Visceral leishmaniasis (VL) persists as a major public health
problem, and since the existing chemotherapy is far from satisfactory,
development of an effective vaccine emerges as the most appropriate strategy
for confronting VL. The development of an effective vaccine relies on the
selection of the appropriate antigen and also the right adjuvant and/or
delivery vehicle. In the present study, the protective efficacy of
poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles (NPs), which were
surface-modified with a TNFα-mimicking eight-amino-acid peptide (p8) and
further functionalized by encapsulating soluble Leishmania infantum antigens
(sLiAg) and monophosphoryl lipid A (MPLA), a TLR4 ligand, was evaluated against
challenge with L. infantum parasites
in BALB/c mice. Vaccination with these multifunctionalized PLGA
nanoformulations conferred significant protection against parasite infection in
vaccinated mice. In particular, vaccination with PLGA-sLiAg-MPLA or
p8-PLGA-sLiAg NPs resulted in almost complete elimination of the parasite in
the spleen for up to 4 months post-challenge. Parasite burden reduction was
accompanied by antigen-specific humoral and cellular immune responses.
Specifically, injection with PLGA-sLiAg-MPLA raised exclusively anti-sLiAg IgG1
antibodies post-vaccination, while in p8-PLGA-sLiAg-vaccinated mice, no
antibody production was detected. However, 4 months post-challenge, in mice
vaccinated with all the multifunctionalized NPs, antibody class switching towards
IgG2a subtype was observed. The study of cellular immune responses revealed the
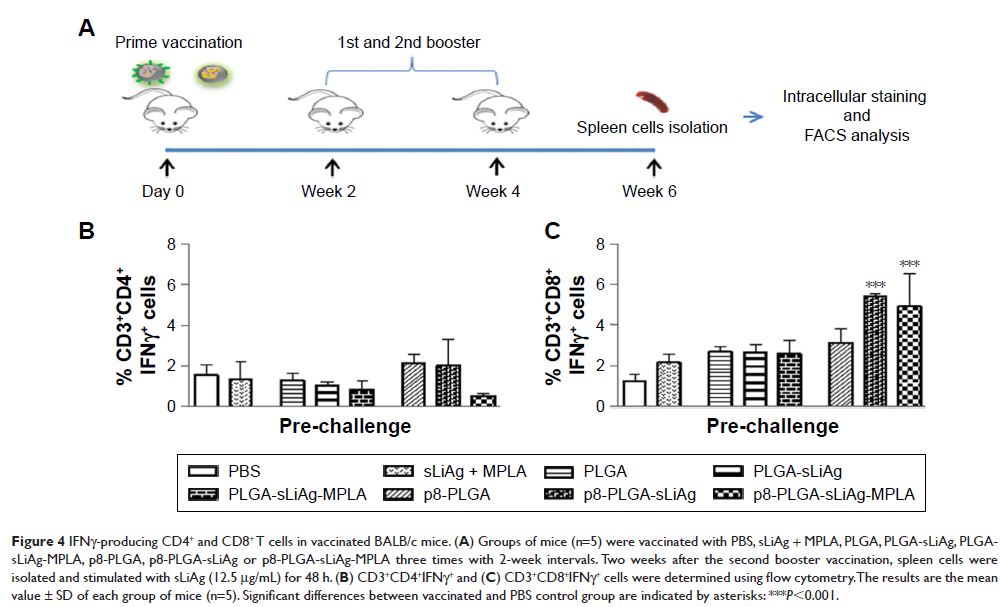
increased proliferation capacity of spleen cells against sLiAg, consisting of
IFNγ-producing CD4+ and CD8+ T cells. Importantly, the activation of CD8+ T cells was exclusively attributed to
vaccination with PLGA NPs surface-modified with the p8 peptide. Moreover,
characterization of cytokine production in vaccinated–infected mice revealed
that protection was accompanied by significant increase of IFNγ and lower
levels of IL-4 and IL-10 in protected mice when compared to control infected
group. Conclusively, the above nanoformulations hold promise for future
vaccination strategies against VL.
Keywords: nanovaccine,
soluble Leishmania antigen, visceral
leishmaniasis, immune response, T cells, cytokines