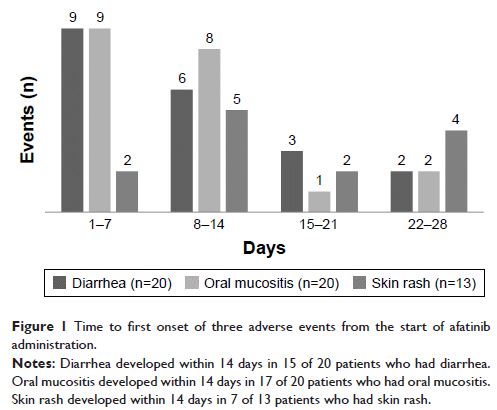
111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Preventive effect of kampo medicine (hangeshashin-to, TJ-14) plus minocycline against afatinib-induced diarrhea and skin rash in patients with non-small cell lung cancer
Authors Ichiki M, Wataya H, Yamada K, Tsuruta N, Takeoka H, Okayama Y, Sasaki J, Hoshino T
Received 6 July 2017
Accepted for publication 4 October 2017
Published 24 October 2017 Volume 2017:10 Pages 5107—5113
DOI https://doi.org/10.2147/OTT.S145613
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 4
Editor who approved publication: Dr Ingrid Espinoza
Purpose: Diarrhea and oral mucositis induced by afatinib can cause
devastating quality of life issues for patients undergoing afatinib treatment.
Several studies have shown that hangeshashin-to (TJ-14) might be useful for
chemotherapy-induced diarrhea and oral mucositis. In this study, we
investigated the prophylactic effects of TJ-14 for afatinib-induced diarrhea
and oral mucositis and minocycline for afatinib-induced skin rash.
Patients and
methods: First- and second-generation
epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors have become
the standard first-line treatment in patients with EGFR-mutated non-small cell
lung cancer. The incidence of diarrhea was higher with afatinib than with gefitinib,
and we conducted a single-arm Phase II study with afatinib. Patients who had
previously undergone treatment with afatinib were ineligible. Both TJ-14
(7.5 g/day) and minocycline (100 mg/day) were administered
simultaneously from the start of afatinib administration. The primary end point
was the incidence of ≥ grade 3 (G3) diarrhea (increase of ≥7 stools/day
over baseline) during the first 4 weeks of treatment. The secondary end
points were the incidence of ≥ G3 oral mucositis (severe pain interfering with
oral intake) and ≥ G3 skin toxicity (severe or medically significant but not
immediately life-threatening).
Results: A total of 29 patients (nine men and 20 women; median age,
66 years; performance status, 0/1/2: 18/10/1) were enrolled from four
centers. Four patients had undergone prior treatment with chemotherapy,
including gefitinib or erlotinib. In all, 20 (68.9%) patients and one (3.4%)
patient had diarrhea of any grade and ≥ G3, respectively. One (3.4%) patient
had ≥ G3 oral mucositis; no patients had ≥ G3 skin rash. A total of 18 (62%) of
the 29 patients achieved a partial response.
Conclusion: The present study indicated a trend in which TJ-14 reduced the
risk of afatinib-induced diarrhea and minocycline reduced the risk of
afatinib-induced skin rash.
Keywords: epidermal growth factor receptor, hangeshashin-to, afatinib,
adverse events