108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

聚天冬氨酸锚定介孔二氧化硅纳米粒子用于 pH 敏感的多柔比星释放
Authors Hakeem A, Zahid F, Zhan G, Yi P, Yang H, Gan L, Yang X
Received 21 July 2017
Accepted for publication 7 December 2017
Published 19 February 2018 Volume 2018:13 Pages 1029—1040
DOI https://doi.org/10.2147/IJN.S146955
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Background: Nanotechnology-based drug delivery systems exhibit promising therapeutic
efficacy in cancer chemotherapy. However, ideal nano drug carriers are supposed
to be sufficiently internalized into cancer cells and then release therapeutic
cargoes in response to certain intracellular stimuli, which has never been an
easy task to achieve.
Objective: This study is to design mesoporous silica nanoparticles (MSNs)-based
pH-responsive nano drug delivery system that is effectively internalized into
cancer cells and then release drug in response to lysosomal/endosomal acidified
environment.
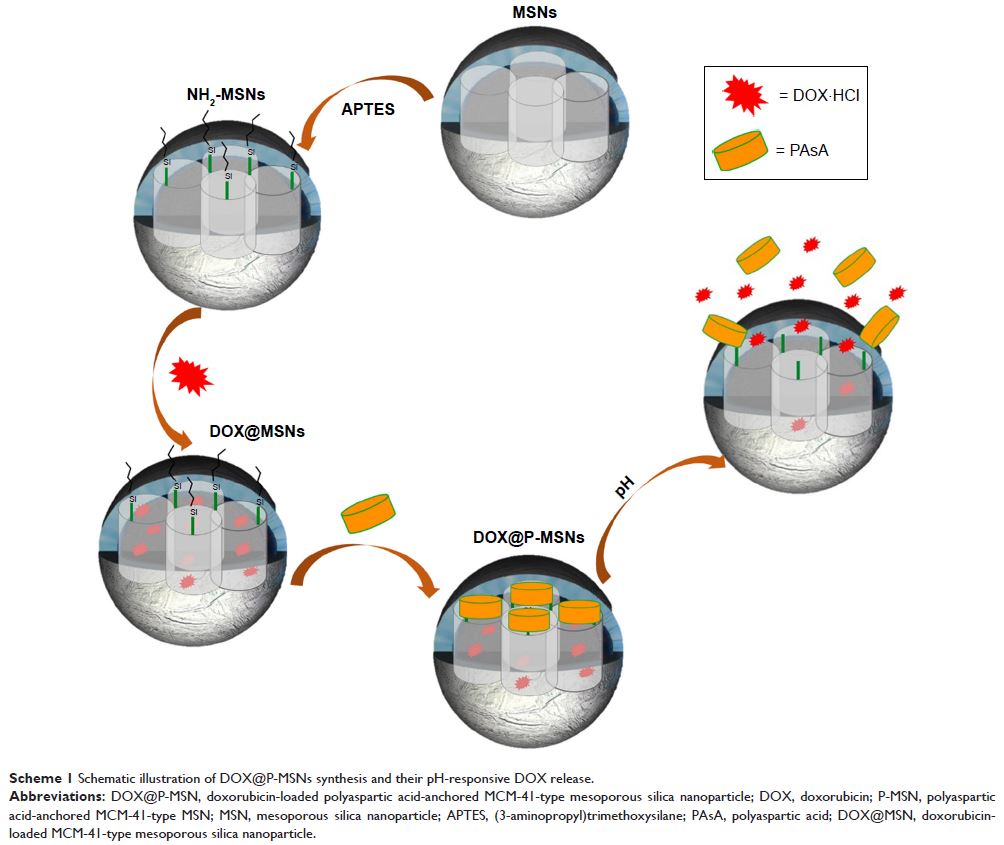
Methods: We synthesized MSNs by sol-gel method. Doxorubicin (DOX) was
encapsulated into the pores as a model drug. Polyaspartic acid (PAsA) was
anchored on the surface of mesoporous MSNs (P-MSNs) as a gatekeeper via amide
linkage and endowed MSNs with positive charge.
Results: In vitro release analysis demonstrated enhanced DOX release from
DOX-loaded PAsA-anchored MSNs (DOX@P-MSNs) under endosomal/lysosomal acidic pH
condition. Moreover, more DOX@P-MSNs were internalized into HepG2 cells than
DOX-loaded MSNs (DOX@MSNs) and free DOX revealed by flow cytometry. Likewise,
confocal microscopic images revealed that DOX@P-MSNs effectively released DOX
and translocated to the nucleus. Much stronger cytotoxicity of DOX@P-MSNs
against HepG2 cells was observed compared with DOX@MSNs and free DOX.
Conclusion: DOX@P-MSNs were successfully fabricated and achieved pH-responsive
DOX release. We anticipated this nanotherapeutics might be suitable contenders
for future in vivo cancer chemotherapeutic applications.
Keywords: cancer chemotherapy, mesoporous silica nanoparticles, polyaspartic
acid, pH-responsive release, cytotoxicity