108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

乌苯美司作为抗癌药物的作用及其与 Akt 抑制剂在人类 A375 和 A2058 细胞中的协同作用
Authors Wang X, Liu Y, Wu R, Guo F, Zhang L, Cui M, Wu X, Zhang Y, Liu W
Received 19 November 2017
Accepted for publication 12 January 2018
Published 22 February 2018 Volume 2018:11 Pages 943—953
DOI https://doi.org/10.2147/OTT.S157480
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Carlos Vigil Gonzales
Background: Malignant
melanoma (MM) is a malignant tumor produced by changes in melanocytes in the
skin or other organs. In the classification of skin tumor mortality, skin
melanoma ranks the highest. Ubenimex, an Aminopeptidase N (APN) inhibitor, is
now widely used for cancer as an adjunct therapy, conferring antitumor effects.
Apoptosis and the induction of autophagy have both been found to be closely
associated with tumor cell death.
Methods: In this study, the A375 and A2058 cell lines were treated with
ubenimex. Cell viability was measured using the Cell Counting Kit 8 assay.
Apoptosis and autophagic cell death were assessed using flow cytometry and
acridine orange/ethidium bromide staining. Protein expression was assessed by
Western blot analyses and immunofluorescence. Matrigel invasion and migration
assays were used to examine the metastatic ability of melanoma cells.
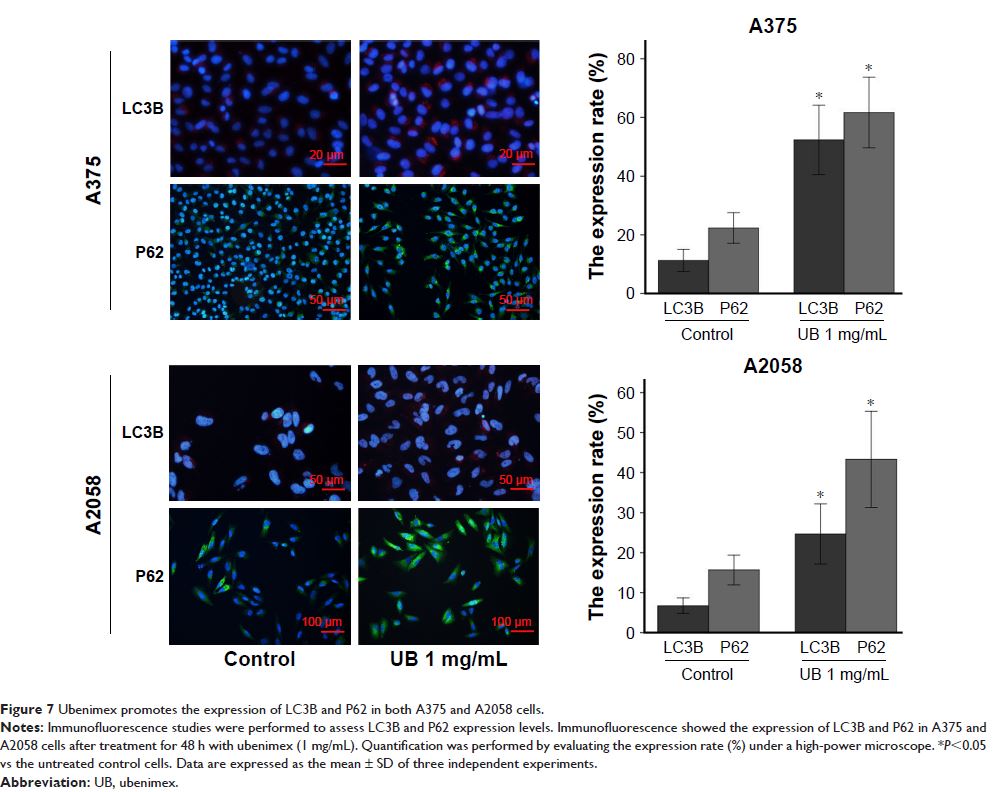
Results: The results revealed that ubenimex inhibited the expression of APN
in melanoma cells, which may be connected with the inhibition of metastasis. In
addition, it increased melanoma cell death by inducing apoptosis and autophagic
cell death. This effect was accompanied by increased levels of p-JNK. Moreover,
treatment with ubenimex induced protective Akt activation, and combined use of
an Akt inhibitor with ubenimex provided a better effect for inducing tumor cell
death.
Conclusion: As an effective anti-tumor drug in vitro, ubenimex might be an
excellent adjunctive therapy for the treatment of melanoma, with greater
effects when combined with the use of an Akt inhibitor.
Keywords: melanoma, ubenimex, jnk, Akt, mixed cell death, metastasis