108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

比较 “左旋多巴/卡比多巴/恩他卡朋” 与 “左旋多巴/多巴 - 脱羧酶抑制剂” 分别用于帕金森病治疗的疗效:系统评价、荟萃分析和经济评价
Authors Yi ZM, Qiu TT, Zhang Y, Liu N, Zhai SD
Received 19 January 2018
Accepted for publication 28 February 2018
Published 16 April 2018 Volume 2018:14 Pages 709—719
DOI https://doi.org/10.2147/TCRM.S163190
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Aims: To review the evidence
for efficacy, safety, and cost-effectiveness of levodopa/carbidopa/entacapone
(LCE) compared with levodopa/dopa-decarboxyiase inhibitor (DDCI) for
Parkinson’s disease (PD).
Methods: PubMed, Embase, the
Cochrane Library, and Chinese databases WangFang Data, Chinese Sci-tech
Journals Database and China National Knowledge Infrastructure, as well
as ClinicalTrials.gov, were searched for
randomized controlled trials with “levodopa/carbidopa/entacapone” as keywords.
The search period was from inception to August 2017. We conducted meta-analyses
to synthesize the evidence quantitatively.
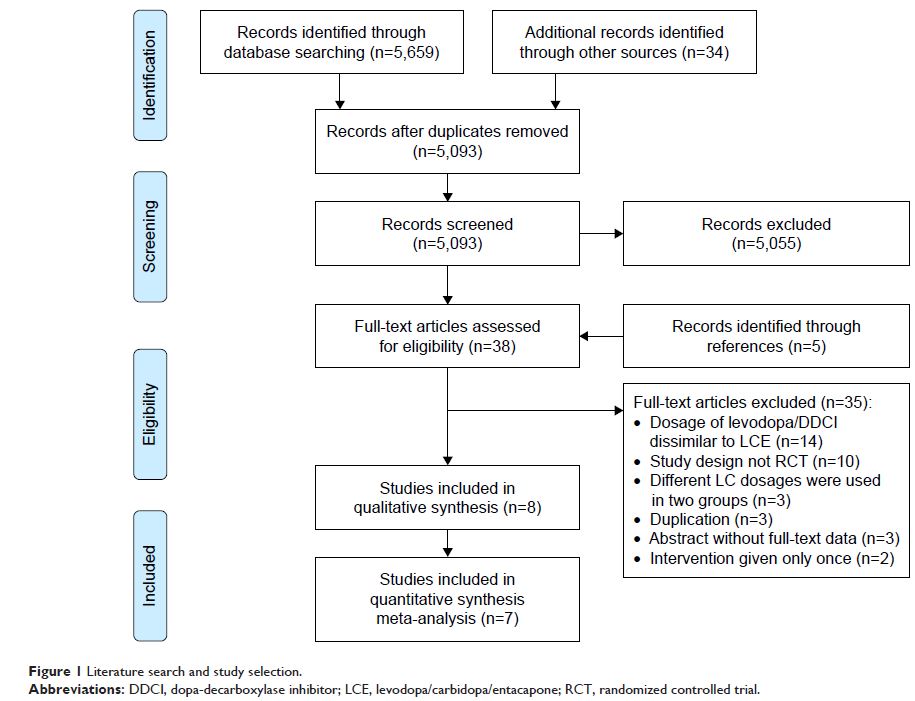
Results: A total of 5,693
records were obtained. We included seven randomized controlled trials and one
cost-effectiveness study after the screening process. Compared with
levodopa–DDCI, LCE improved patient Unified Parkinson’s Disease Rating Scale
(UPDRS) II score (mean difference [MD] -1.17, 95% CI -1.64 to -0.71), UPDRS III
score (MD -1.55, 95% CI -2.29 to -0.81), and Schwab and England daily activity
rating (MD 2.05, 95% CI 0.85–3.26). There was no statistically significant
difference in the risk of serious adverse events (AEs) or discontinuation due
to AEs in patients with LCE, and the risk of total AEs was higher in the LCE
group (risk ratio [RR] 1.33, 95% CI 1.05–1.70). The incremental
cost-effectiveness ratio of LCE was £3,105 per quality-adjusted life-year
(QALY) gained in the UK.
Conclusion: LCE can improve PD
patients’ motor symptoms and daily living functioning when compared with levodopa/DDCI.
Keywords: Unified Parkinson’s
Disease Rating Scale, quality of life, wearing off, adverse events,
cost-effectiveness, health technology assessment