108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials
Authors Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N
Received 19 December 2017
Accepted for publication 27 February 2018
Published 3 May 2018 Volume 2018:11 Pages 923—934
DOI https://doi.org/10.2147/JPR.S160255
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Introduction: Opioids have been used for millennia for the treatment of pain.
However, the long-term efficacy of opioids to treat chronic non-cancer pain
continues to be debated. To evaluate opioids’ efficacy in chronic non-cancer
pain, we performed a meta-analysis of published clinical trials for μ-opioid
receptor agonists performed for US Food and Drug Administration approval.
Methods: MEDLINE and Cochrane trial register were
searched for enriched enrollment randomized withdrawal studies (before June
2016). Selection criteria included: adults, ≥10 subjects per arm, any chronic
pain condition, double-blind treatment period lasting ≥12 weeks, and all
μ-agonist opioids approved in the USA.
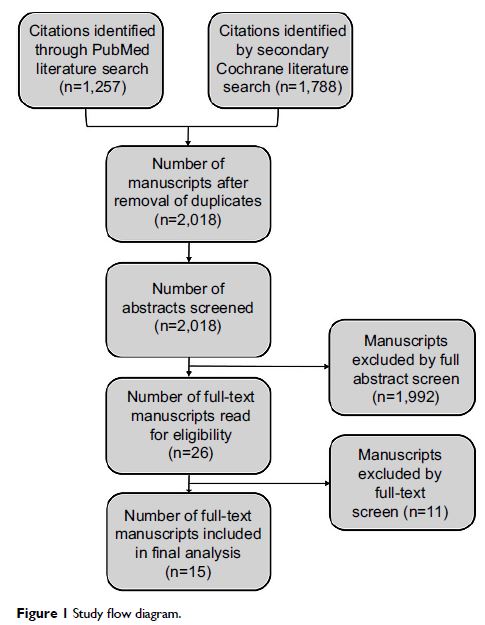
Results: Fifteen studies met criteria. Opioid efficacy
was statistically significant (p <0.001) versus
placebo for pain intensity (standardized mean difference: −0.416), ≥30% and
≥50% improvement in pain (risk difference: 0.166 and 0.137), patient global
impression of change (0.163), and patient global assessment of study medication
(0.194). There were minor benefits on physical function and no effect on mental
function.
Conclusion: Opioids are efficacious in the treatment of
chronic non-cancer pain for up to 3 months in randomized controlled trials.
This should be considered, alongside data on opioid safety, in the use of
opioids for the treatment of chronic pain.
Keywords: opioid
analgesics, non-cancer pain, long-term efficacy, EERW trials, opioid efficacy;
evidence-based medicine