108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过颅内注射递送贝伐单抗: 在胶质瘤模型中进行的评估
Authors Liu YX, Liu WJ, Zhang HR, Zhang ZW
Received 14 December 2017
Accepted for publication 20 March 2018
Published 8 May 2018 Volume 2018:11 Pages 2673—2683
DOI https://doi.org/10.2147/OTT.S159913
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Ingrid Espinoza
Background: Many
reports have indicated that the intravenous administration of bevacizumab
produces a number of systemic side effects. Therefore, we investigated the
therapeutic effects of intratumoral bevacizumab administration using a glioma
animal model.
Methods: The glioma cell lines U251 and U87 that carried luciferase were
implanted into the brains of mice to develop glioma models. Glioma-bearing mice
were treated with bevacizumab intravenously or intratumorally by Alzet
micro-osmotic pumps, and the survival time of mice was monitored. Tumor volumes
and location were observed by fluorescence imaging and histological analysis.
Levels of microvessel marker, cancer stem cell marker as well as angiogenesis-,
invasion-, and inflammation-related factors in tumors were examined by
immunohistochemical staining.
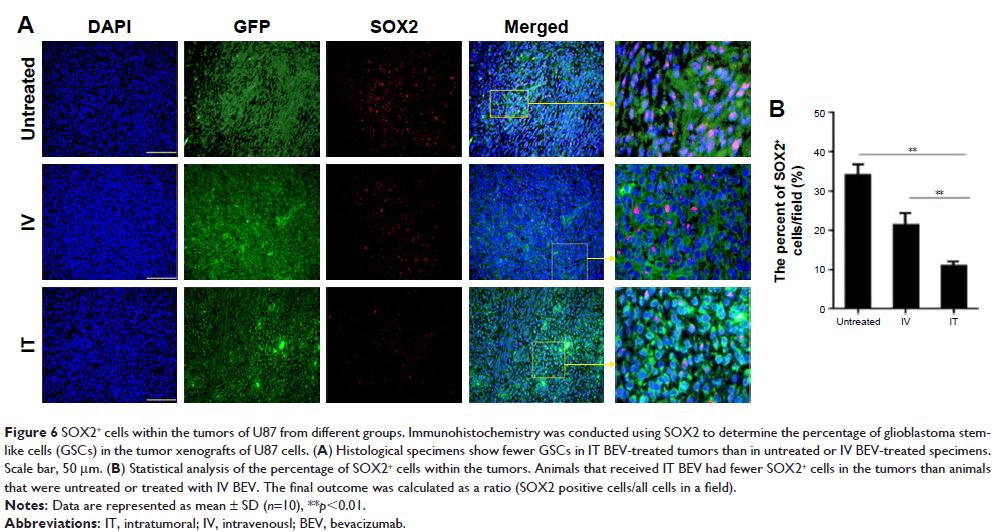
Results: Mice treated with intratumoral low-dose bevacizumab had smaller
tumor volumes, longer survival time, lower microvessel density, and fewer
cancer stem cells as compared with untreated and intravenously treated mice.
Furthermore, expression levels of inflammation-related factors increased
signifiwhereas that of angiogenesis- and invasion-related factors decreased in
intratumorally treated animals, compared with intravenously treated mice.
Conclusion: These results implied bevacizumab delivery by intratumoral
injection via Alzet micro-osmotic pumps may be a more effective and safer
protocol for treating gliomas.
Keywords: bevacizumab, anti-angiogenic, glioma cell line, intratumoral
delivery, fluorescence imaging