108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CPT2 的下调可促进肝细胞癌肿瘤形成和对顺铂的化疗耐药性
Authors Lin MH, Lv D, Zheng YL, Wu ML, Xu C, Zhang Q, Wu LH
Received 21 January 2018
Accepted for publication 26 March 2018
Published 25 May 2018 Volume 2018:11 Pages 3101—3110
DOI https://doi.org/10.2147/OTT.S163266
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Cancer cells often have characteristic changes in metabolism.
Besides Warburg effect, abnormal lipid metabolism is also considered as one of
the most typical metabolic symbols of cancer. Thus, understanding the
mechanisms of cell metabolic reprogramming may provide a potential avenue for
cancer treatment.
Materials and
methods: In total, 41 pairs of matched
samples of primary hepatocellular carcinoma (HCC) and adjacent non-cancerous
liver tissues were collected. Afterward, we performed quantitative reverse
transcriptase polymerase chain reaction to investigate carnitine
palmitoyltransferase-2 (CPT2) expression and then systematically analyzed its
relationship with clinicopathologic features. We further performed
proliferation, colony formation, migration and invasion, drug resistance, and
lipogenesis assays to determine the function of CPT2 in HCC.
Results: In this study, we have identified CPT2 which is the rate-limiting
enzyme of fatty acid oxidation, downregulated in HCC and was significantly
associated with tumor histological differentiation and venous invasion. In
vitro studies demonstrated that knockdown of CPT2 remarkably enhanced the
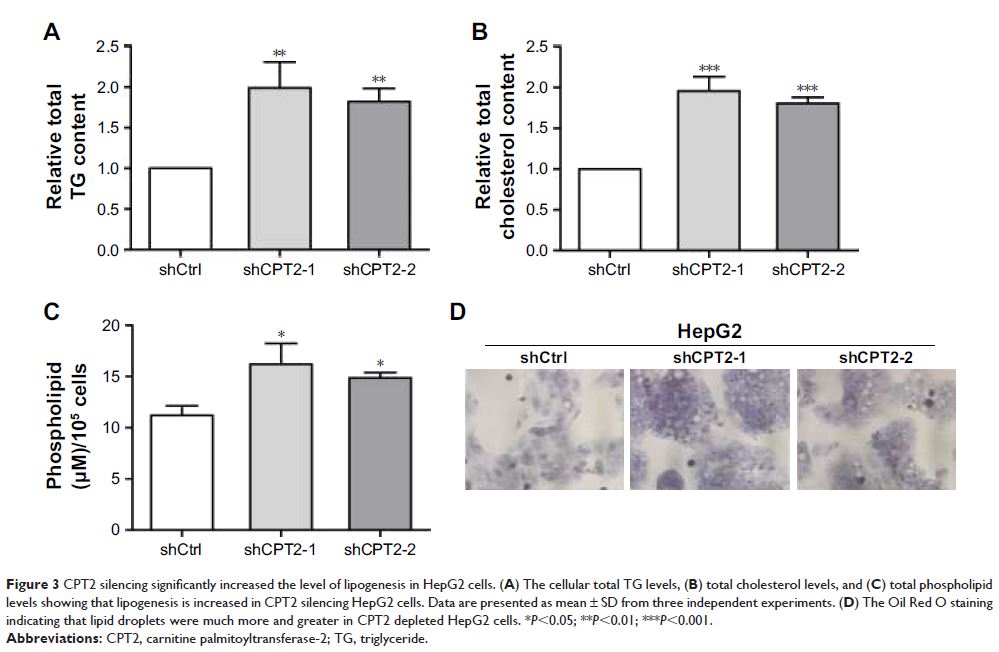
tumorigenic activity and metastatic potential of hepatoma cells. In addition,
CPT2 silencing induced chemoresistance to cisplatin. Mechanistically, low
expression of CPT2 promoted cancer cell lipogenesis via upregulation of
stearoyl-CoA desaturase-1, the key enzyme involved in the synthesis of
monounsaturated fatty acids, at both mRNA and protein levels in hepatoma cell
line.
Conclusion: Altogether, our findings demonstrate that CPT2 has a critical role
in HCC progression and chemoresistance and may potentially serve as a novel
prognostic marker and therapeutic target for HCC treatment.
Keywords: lipid metabolism, CPT2, HCC, SCD1, chemoresistance