108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

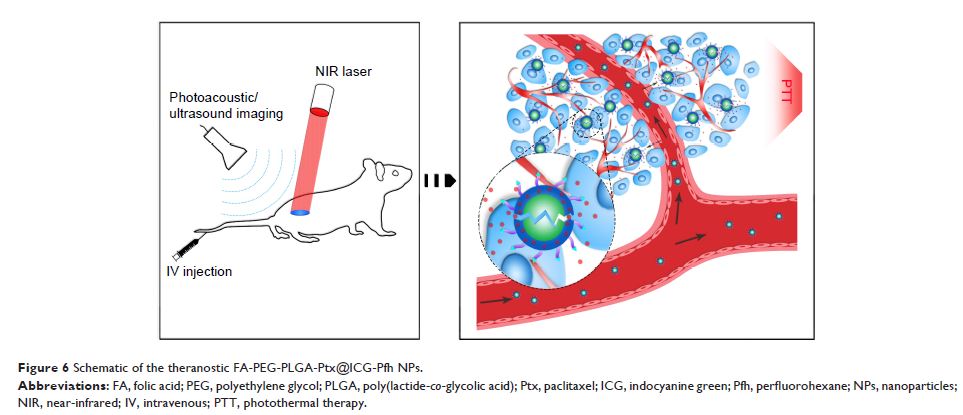
叶酸受体靶向的激光活化聚(丙交酯 -co - 乙醇酸)纳米粒子(负载紫杉醇/吲哚青绿)可用于光声/超声成像和化学/光热疗法
Authors Liu F, Chen Y, Li Y, Guo Y, Cao Y, Li P, Wang Z, Gong Y, Ran H
Received 1 March 2018
Accepted for publication 18 June 2018
Published 6 September 2018 Volume 2018:13 Pages 5139—5158
DOI https://doi.org/10.2147/IJN.S167043
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Thiruganesh Ramasamy
Peer reviewer comments 3
Editor who approved publication: Dr Lei Yang
Background: Cancer is one of the most serious threats to human health. Precision medicine is an innovative approach to treatment, as part of which theranostic nanomedicine has been studied extensively. However, the required biocompatibility and substantial cost for the approval of nanomedicines hinder their clinical translation.
Purpose: We designed a novel type of theranostic nanoparticle (NP) folate-receptor-targeted laser-activatable poly(lactide-co -glycolic acid) (PLGA) NPs loaded with paclitaxel (Ptx)/indocyanine green (ICG)-folic acid-polyethylene glycol (PEG)-PLGA-Ptx@ICG-perfluorohexane (Pfh)- using safe and approved materials and drugs, which would facilitate clinical translation. With laser irradiation, highly efficient photothermal therapy can be achieved. Additionally, targeted NPs can be activated by near-infrared laser irradiation at a specific region, which leads to the sharp release of Ptx at areas of high folate-receptor expression and ensures a higher Ptx concentration within the tumor region, thereby leading to chemo/photothermal synergistic antitumor efficacy. Meanwhile, the NPs can be used as a dual-modality contrast agent for photoacoustic and ultrasound imaging.
Materials and methods: FA-PEG-PLGA-Ptx@ICG-Pfh NPs were prepared by sonification method and characterized for physicochemical properties. Cytotoxicity and in vivo biocompatibility were evaluated respectively by CCK8 assay and blood analysis. NPs as dual-modality contrast agents were evaluated by photoacoustic/ultrasound imaging system in vitro and in vivo. In vitro anticancer effect and in vivo anticancer therapy was evaluated by CCK8 assay and MDA-MB231 tumor-bearing mice model.
Results: FA-PEG-PLGA-Ptx@ICG-Pfh NPs were in the size of 308±5.82 nm with negative zeta potential and showed excellent photothermal effect. The NPs could be triggered sharp release of Ptx by laser irradiation, and showed the good biocompatibility in vitro and in vivo. Through photoacoustic/ultrasound imaging, the NPs showed an excellent ability as dual-modality contrast agents in vitro and in vivo. FA-PEG-PLGA-Ptx@ICG-Pfh NPs with laser irradiation showed the best anticancer efficacy in vitro and in vivo.
Conclusion: Such a biocompatible and novel theranostic NP is expected to integrate dual-modality imaging with improved therapeutic efficacy and provide a promising paradigm for cancer therapy.
Keywords: nanomedicine, folate-receptor-targeted nanoparticle, theranostics, targeted drug-delivery system, combined anticancer therapy, photoacoustic imaging, ultrasound imaging