108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

RRM1 表达与接受吉西他滨治疗的非小细胞肺癌患者的临床病理特征
Authors Chen Y, Huang Y, Chen DM, Wu C, Leng QP, Wang WY, Deng MQ, Zhao YX, Yang XH
Received 16 January 2018
Accepted for publication 9 July 2018
Published 7 September 2018 Volume 2018:11 Pages 5579—5589
DOI https://doi.org/10.2147/OTT.S162667
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: The usefulness of ribonucleotide reductase catalytic subunit M1 (RRM1) for predicting the therapeutic effects of gemcitabine-containing chemotherapy in patients with non-small cell lung cancer (NSCLC) remains controversial. RRM1-positive patients show unique clinicopathological features.
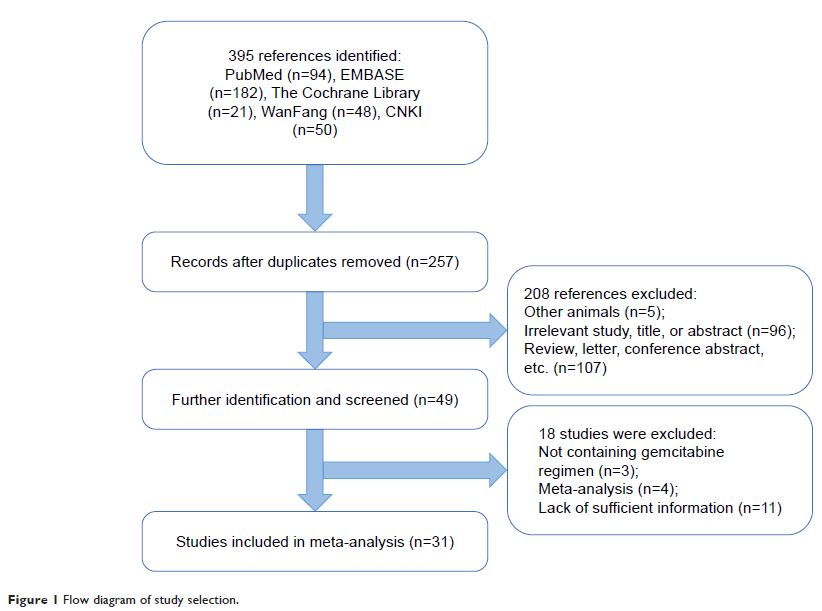
Methods: Here, we performed a meta-analysis to systematically evaluate the relationship between RRM1 expression and the clinicopathological characteristics of NSCLC patients treated with gemcitabine-containing regimens. A comprehensive electronic and manual search was performed to identify relevant articles. The pooled relative risk (RR) and 95% CI were used to estimate the relation between the clinicopathological characteristics of NSCLC patients and RRM1 expression.
Results: The study included 31 observational studies and 3,667 patients. The analysis showed no significant association between RRM1 expression and pathological type, stage, and smoking status; however, RRM1 positivity was significantly lower in women than in men (43.0% vs 51.7%, RR=0.84, 95% CI: 0.74–0.94, P =0.004).
Conclusion: The present pooled analyses demonstrated that RRM1 positivity in women with advanced NSCLC was associated with a higher rate of response to gemcitabine-containing regimens. Immunohistochemistry may be valuable to prescreen for RRM1 expression in clinical practice, whereas PCR can be routinely used as a verification method. These findings will help design suitable molecular-targeted therapies for NSCLC.
Keywords: RRM1, gemcitabine, meta-analysis, clinicopathological features, NSCLC