108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

确定结直肠癌患者对 FOLFOX 治疗反应的预测基因
Authors Lin HJ, Qiu XK, Zhang B, Zhang JC
Received 8 March 2018
Accepted for publication 12 July 2018
Published 17 September 2018 Volume 2018:11 Pages 5943—5955
DOI https://doi.org/10.2147/OTT.S167656
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Federico Perche
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Colorectal cancer is a malignant tumor with high death rate.
Chemotherapy, radiotherapy and surgery are the three common treatments of
colorectal cancer. For early colorectal cancer patients, postoperative adjuvant
chemotherapy can reduce the risk of recurrence. For advanced colorectal cancer
patients, palliative chemotherapy can significantly improve the life quality of
patients and prolong survival. FOLFOX is one of the mainstream chemotherapies
in colorectal cancer, however, its response rate is only about 50%.
Methods: To systematically investigate why some of the colorectal cancer
patients have response to FOLFOX therapy while others do not, we searched all
publicly available database and combined three gene expression datasets of
colorectal cancer patients with FOLFOX therapy. With advanced minimal
redundancy maximal relevance and incremental feature selection method, we
identified the biomarker genes.
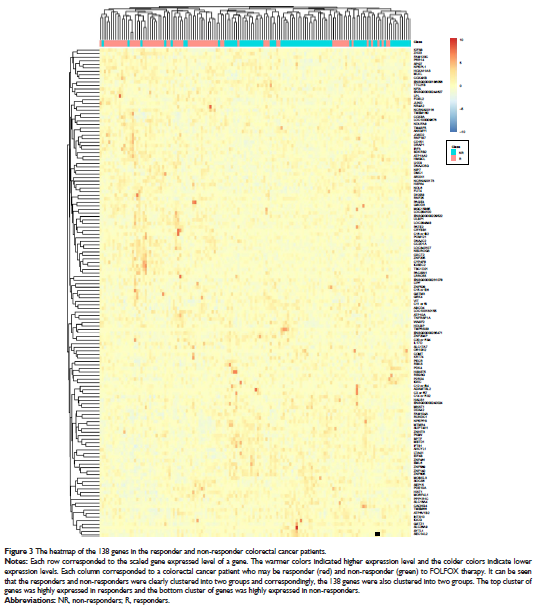
Results: A Support Vector Machine-based classifier was constructed to
predict the response of colorectal cancer patients to FOLFOX therapy. Its
accuracy, sensitivity and specificity were 0.854, 0.845 and 0.863,
respectively.
Conclusion: The biological analysis of representative biomarker genes
suggested that apoptosis and inflammation signaling pathways were essential for
the response of colorectal cancer patients to FOLFOX chemotherapy.
Keywords: colorectal cancer, FOLFOX therapy, support vector machine, minimal
redundancy maximal relevance, incremental feature selection, chemotherapy
response