108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

与聚(ADP-核糖)聚合酶(PARP)抑制剂相关的选择性胃肠道毒性在卵巢癌治疗中的风险:关于已发表试验的一项综合分析
Authors Liu Y, Meng J, Wang G
Received 4 February 2018
Accepted for publication 10 May 2018
Published 17 September 2018 Volume 2018:12 Pages 3013—3019
DOI https://doi.org/10.2147/DDDT.S164553
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Palas Chanda
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Aims: We aimed to comprehensively assess the risk of gastrointestinal
toxicities associated with poly (ADP-ribose) polymerase inhibitors (PARPis) in
the treatment of ovarian cancer patients.
Materials and
methods: We searched several databases for
relevant trials. Eligible studies included prospective Phase II and III trials
of ovarian cancer patients on the four PARPis (olaparib, veliparib, niraparib
and rucaparib), describing events of nausea, vomiting, diarrhea, and constipation.
Summary incidence, relative risk (RR), and 95% CIs were calculated
employing fixed- or random-effects models.
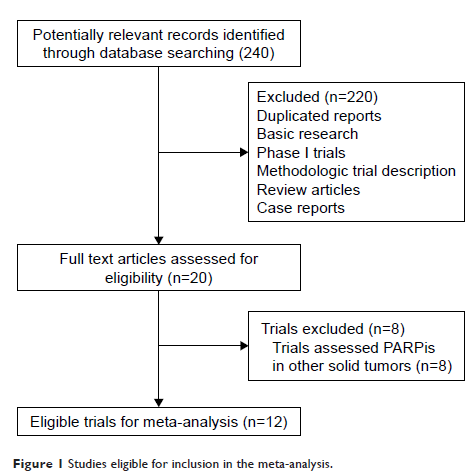
Results: A total of 2,286 ovarian cancer patients from 12 trials were
included for analysis. Our results showed that summary incidences of all-grade
gastrointestinal events in ovarian cancer patients were nausea 68.8% (95% CI,
63.5%–73.6%), vomiting 36.2% (95% CI, 30.9%–41.8%), diarrhea 25.3% (95% CI,
21.2%–29.8%), and constipation 25.3% (95% CI, 17.9%–34.5%). The RRs of
all-grade nausea, vomiting, diarrhea, and constipation were 2.00 (95% CI:
1.79–2.24; P <0.001), 2.12 (95% CI:
1.75–2.58; P <0.001), 1.20 (95% CI:
1.01–1.44; P =0.044), and 1.20 (95% CI:
0.88–1.80; P =0.21); respectively. While, the
RRs of high-grade nausea, vomiting, diarrhea, and constipation were 3.74 (95%
CI: 1.50–9.36; P =0.005), 2.81 (95%
CI: 1.17–6.74; P =0.02), 0.56 (95%
CI: 0.22–1.43; P =0.23), 0.92 (95%
CI: 0.34–2.49, P =0.87);
respectively.
Conclusion: Our study suggests that the risk of all-grade gastrointestinal
toxicities associated with PARPis, excepting constipation, is significantly
increased in ovarian cancer patients. And the use of PARPis significantly
increased the risk of developing high-grade nausea and vomiting, but not for
diarrhea and constipation. Close clinical monitoring is recommended when
administering these drugs.
Keywords: poly (ADP-ribose) polymerase inhibitors, gastrointestinal
toxicities, clinical trials, meta-analysis, targeted agents, gynaecological
tumors, systematic review