111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在模拟的肿瘤微环境中,膀胱癌和 T 细胞谷氨酸升高可促进干扰素-γ 引起的 PD-L1 表达上调
Authors Wang LP, Yang XC, Li D, Liang ZJ, Chen YB, Ma GF, Wang YH, Li YX, Liang Y, Niu HT
Received 17 July 2018
Accepted for publication 10 September 2018
Published 18 October 2018 Volume 2018:11 Pages 7229—7243
DOI https://doi.org/10.2147/OTT.S180505
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Background: Metabolic reprogramming occurs in the tumor microenvironment and
influences the survival and function of tumor and immune cells. Interferon-γ
(IFN-γ) produced by T cells up-regulates PD-L1 expression in tumors. However,
reports regarding the relationship between nutrient metabolism and the
up-regulation of PD-L1 expression are lacking.
Materials and
methods: In this paper, we analyzed the
metabolic changes in T cells and bladder cancer cells in a simulated tumor
microenvironment to provide evidence regarding their relevance to PD-L1 up-regulation.
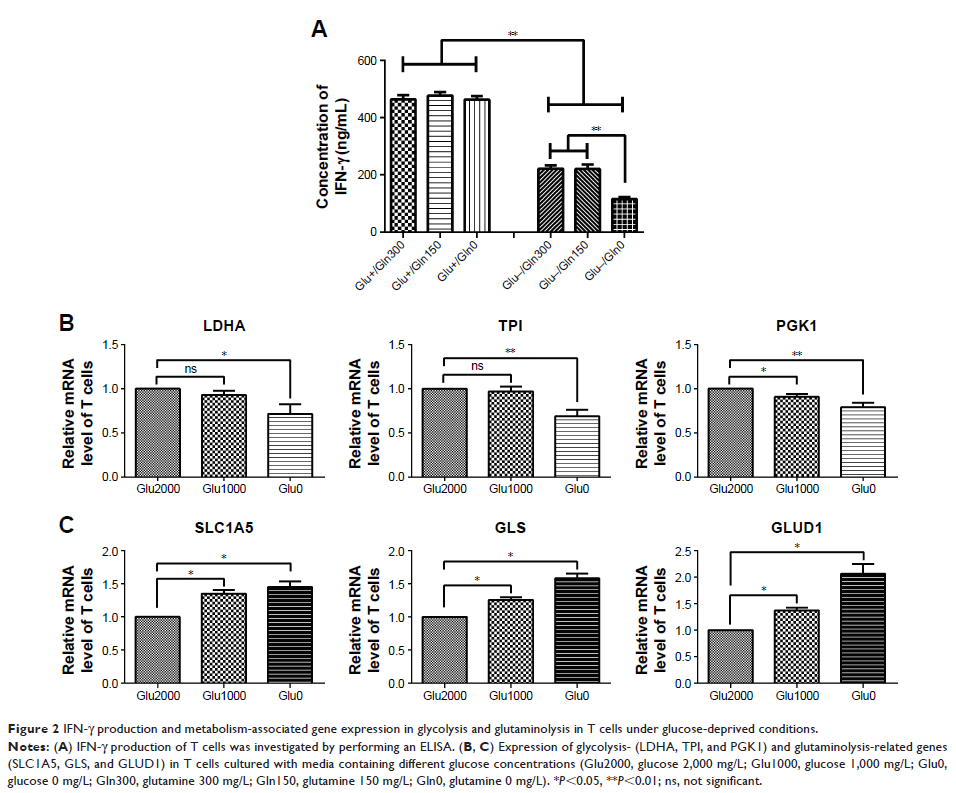
Results: The glutaminolysis was increased in both activated T cells and
glucose-deprived T cells. IFN-γ production by T cells was decreased in a
glucose-free medium and severely decreased when cells were simultaneously
deprived of glutamine. Furthermore, the glutaminolysis of the bladder cancer
cells under glucose deprivation exhibited a compensatory elevation. The glucose
concentration of T cells co-cultured with bladder cancer cells was decreased
and T cell proliferation was reduced, but IFN-γ production and glutaminolysis
were increased. However, in bladder cancer cells, the elevation in
glutaminolysis under co-culture conditions did not compensate for glucose
deprivation because the glucose concentration in the culture medium did not
significantly differ between the cultures with and without T cells. Our data
also show that inhibiting glutamine metabolism in bladder cancer cells could
reduce the elevation in PD-L1 expression induced by IFN-γ.
Conclusion: In a simulated tumor microenvironment, elevated glutaminolysis may
play an essential role in IFN-γ production by T cells, ultimately improving the
high PD-L1 expression, and also directly contributing to producing more PD-L1
in bladder cancer cells.
Keywords: T cells, bladder cancer cells, glutaminolysis, PD-L1, co-culture