110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

胰岛素磷脂复合物可变形纳米囊泡增强胰岛素口腔递送效果的机制
Authors Xu Y, Zhang X, Zhang Y, Ye J, Wang HL, Xia X, Liu Y
Received 28 May 2018
Accepted for publication 14 August 2018
Published 9 November 2018 Volume 2018:13 Pages 7319—7331
DOI https://doi.org/10.2147/IJN.S175425
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 4
Editor who approved publication: Dr Linlin Sun
Background: Non-injectable delivery of peptides and proteins are not feasible
due to its large molecular, high hydrophilic and gastrointestinal degradation.
Therefore, proposing a new method to solve this problem is a burning issue.
Purpose: The objective of this study was to propose a
novel protein delivery strategy to vanquish the poor efficacy of buccal mucosa
delivery systems for protein delivery and then investigate the detailed
mechanisms of the enhanced buccal delivery of protein, using insulin as a model
drug.
Materials and methods: Insulin-phospholipid complex combined with
deformable nanovesicles (IPC-DNVs) were prepared, using deformable nanovesicles
based on insulin (INS-DNVs) and conventional nanovesicles based on insulin-phospholipid
complex (IPC-NVs) as references. Besides, their physicochemical
characterization, in vitro transport behavior, in vivo bioactivity and
hypoglycemic effect were systematically characterized and compared. Finally, we
evaluated the in vivo safety of IPC-DNVs.
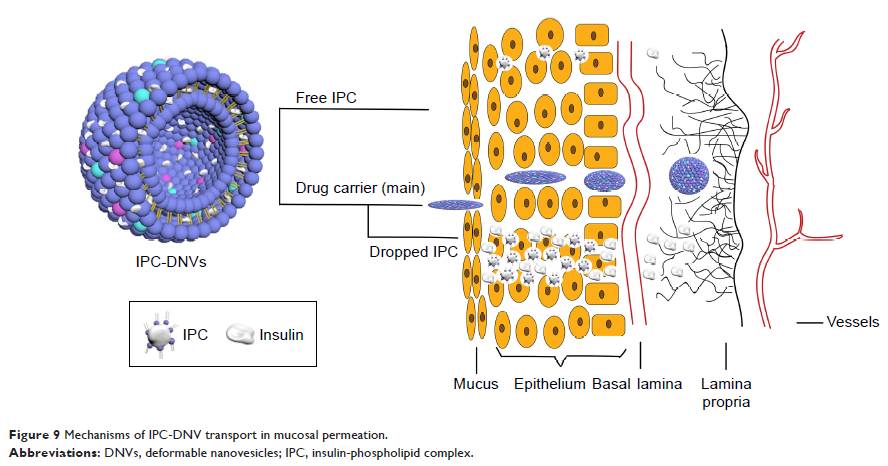
Results: First, IPC-DNVs increased insulin permeability through
deposition of the IPC and deformability of the DNVs, which was revealed by an
in vitro mucosal permeation study. Second, DNVs could act as a drug carrier and
penetrate the mucosa to reach the receiver medium as intact nanovesicles, which
was supported by the observation of intact nanovesicles in the receiver medium
through transmission electron microscopy (TEM). Third, IPC-DNVs exhibited both
transcellular and paracellular transport in the form of IPC and DNVs,
respectively, which was proved by confocal laser scanning microscopy (CLSM).
Unlike the other two formulations, IPC-DNVs exhibited a sustained mild
hypoglycemic effect, with a relative bioavailability (Fp) of 15.53% (3.09% and
1.96% for INS-DNVs and IPC-NVs, respectively). Furthermore, buccal
administration of IPC-DNVs resulted in no visible mucosal irritation to the
buccal mucosa.
Conclusion: Our work reveals the mechanisms underlying the
enhanced buccal delivery of IPC-DNVs: the DNVs facilitate penetration through
the main barrier, and the deposition of IPC enhances buccal absorption. Our
results and proposed mechanisms could be an important reference to understand
other nanocarriers based on protein (peptide)-phospholipid complexes that
penetrate the mucosa and provide a theoretical basis for the future development
of buccal delivery systems for insulin.
Keywords: diabetes,
hypoglycemic effect, mucosal permeation, absorption, safety