110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

新型 pH 敏感性锌酞菁与白蛋白结合用于肿瘤靶向和治疗
Authors Wang Y, Zheng K, Xuan G, Huang M, Xue J
Received 23 July 2018
Accepted for publication 9 October 2018
Published 19 November 2018 Volume 2018:13 Pages 7681—7695
DOI https://doi.org/10.2147/IJN.S181199
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
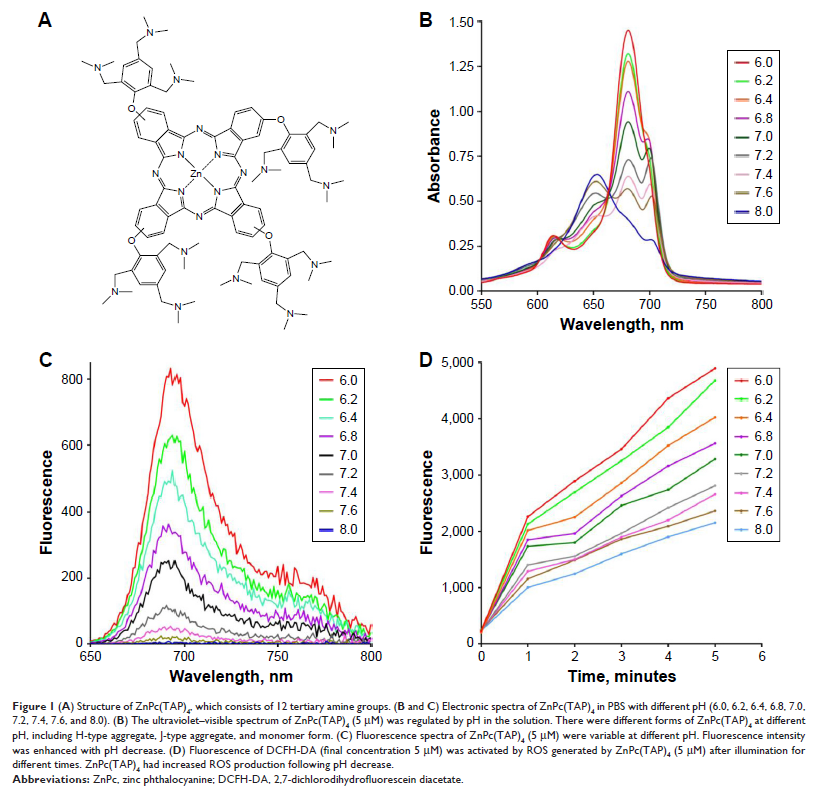
Purpose: Zinc
phthalocyanine (ZnPc) has been applied widely in photodynamic therapy (PDT)
with high ROS-production capacity and
intense absorption in the near-infrared region. However, weak tumor targeting
and the aggregation tendency of ZnPc seriously affect the therapeutic effect of
PDT. Therefore, overcoming the aggregation of ZnPc and enhancing its antitumor
effect were the purpose of this study.
Methods: In this
study, we first found that the aggregation behaviors of the photosensitizer
ZnPc(TAP)4, ZnPc substituted by tertiary amine groups,
were regulated finely by pH and that ZnPc(TAP)4 could be
disaggregated gradually as the pH descended. ZnPc(TAP)4 and human
serum albumin (HSA) molecules were assembled into nanoparticles (NPs) for tumor
targeting. Meanwhile, the chemotherapy drug paclitaxel (Ptx) was loaded into
HSA NPs together with ZnPc(TAP)4 for dual antitumor effects. HSA NPs
loading both ZnPc(TAP)4 and Ptx (NP–ZnPc[TAP]4–Ptx) were
characterized by particle size and in vitro release. Cytotoxicity, subcellular
localization, tumor targeting, and anticancer effect in vivo were investigated
respectively.
Results: We found
that NP–ZnPc(TAP)4–Ptx had good stability with qualifying
particle size. Interestingly, ZnPc(TAP)4 was
released from the NPs and the photodynamic activity enhanced in the acidic
environment of tumor. In addition, NP–ZnPc(TAP)4–Ptx had
prominent cytotoxicity and time-dependent subcellular localization
characteristics. Through a three-dimensional animal imaging system,
NP–ZnPc(TAP)4–Ptx showed much-enhanced tumor targeting in
tumor-bearing mice. Above all, NP–ZnPc(TAP)4–Ptx was
demonstrated to have the synergistic anticancer effect of PDT and chemotherapy.
Conclusion: NP–ZnPc(TAP)4–Ptx had
enhanced tumor targeting for the pH-sensitive property of ZnPc(TAP)4 and the
transport function of HSA. NP–ZnPc(TAP)4–Ptx possessed
a double-anticancer effect through the combination of ZnPc(TAP)4 and Ptx.
This drug-delivery system may also be used to carry chemotherapy drugs other
than Ptx for improving antitumor effects.
Keywords: photodynamic
therapy, drug-delivery system, controlled release, chemotherapy, antitumor
activity, combination therapy