110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

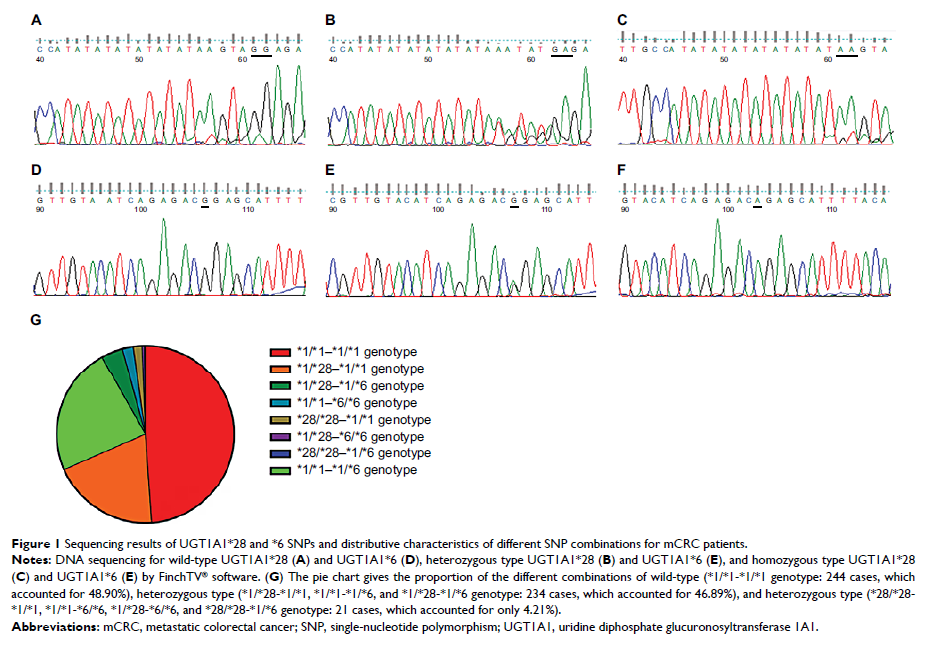
利用伊立替康对杂合型 UGT1A1*6 或 UGT1A1*28 晚期结直肠癌患者进行的个体化化疗中血浆 SN-38 水平和 DPD 活性的价值
Authors Tian C, Ying HF, Zhuang RY, Zhang XW, Lu HM, Wang H, Wang SW, Li Q, Wang CG, Cai X
Received 12 June 2018
Accepted for publication 11 September 2018
Published 22 November 2018 Volume 2018:10 Pages 6217—6226
DOI https://doi.org/10.2147/CMAR.S176918
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Naduparambil K. Jacob
Purpose: The
relationship between the pharmacokinetics of irinotecan and outcomes of
advanced colorectal cancer is unclear, and few studies have examined
individualized irinotecan-based chemotherapy depending on plasma 7-ethyl-10-hydroxy
camptothecin (SN-38) levels and dihydropyrimidine dehydrogenase (DPD) activity,
particularly for the UGT1A1*6 or UGT1A1*28 heterozygous type.
Methods: This study
retrospectively explored the relationship among plasma SN-38 level 1.5 hours
after critical enzyme for irinotecan (CPT-11) administration (CSN-38 1.5h), plasma SN-38
level 49 hours after CPT-11 administration (CSN-38 49h), DPD
activity, and clinical outcomes for the UGT1A1*6 and UGT1A1*28 heterozygous
types.
Results: CSN-38 1.5h and CSN-38 49h of the
UGT1A1*6 or UGT1A1*28 heterozygous type were close to those of UGT1A1*6 and
UGT1A1*28 wild-types; some of those with relatively high CSN-38 1.5h levels
obtained better median progression-free survival (mPFS), whereas others with
higher CSN-38 49h concentrations showed a relatively high
incidence of adverse reactions possibly because of the decreased activity of
DPD.
Conclusion: Increasing
the dosage of CPT-11 according to CSN-38 1.5h may
improve the efficacy in patients with lower CSN-38 1.5h levels.
For cases with comparably low DPD activity, advisable primary and subsequent
dose adjustment of 5-fluorouracil based on plasma 5-fluorouracil levels may be
a practical strategy for reducing the occurrence of adverse reactions for
personalized treatment of the UGT1A1*6 or UGT1A1*28 heterozygous type.
Keywords: irinotecan,
pharmacokinetics, enzyme activity, uridine diphosphate glucuronosyltransferase
1A1, colorectal cancer