110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

羟考酮调节急性手术后疼痛大鼠模型中切口诱导的神经营养因子和受体的激活
Authors Liu BW, Liu Y, Li NB, Zhang J, Zhang XW
Received 16 July 2018
Accepted for publication 14 August 2018
Published 30 October 2018 Volume 2018:11 Pages 2663—2674
DOI https://doi.org/10.2147/JPR.S180396
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Background: Oxycodone, which is one of the most commonly used opiates in postoperative pain management, has a different affinity for μ-opioid receptors (MOR), κ-opioid receptors (KOR), and δ-opioid receptors (DOR). Accumulating research has suggested that neurotrophins (NTs) are involved in opioid analgesia. In the current exploratory study, we aimed to investigate the underlying mechanisms of the analgesic effects of oxycodone on post-surgery pain in rats and to determine whether neurotrophic factors and receptors were involved in these effects.
Methods: Mechanical and thermal sensitivity tests were used to evaluate the validity of the postoperative pain rat model and to determine the analgesic effect of oxycodone. Quantitative PCR and Western blot analysis were used to detect the changes in the expression of three types of opioid receptors and NTs and their high-affinity receptors in the spinal cord after surgery and oxycodone administration.
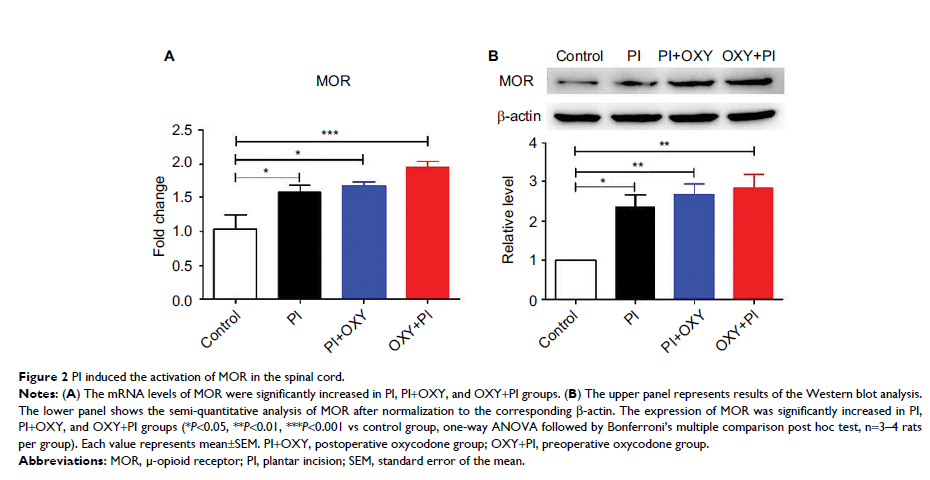
Results: Oxycodone showed an analgesic effect on plantar incision (PI)-induced hyperalgesia, especially thermal hyperalgesia. We detected an obvious increase in MOR expression levels but insignificant changes in KOR and DOR levels in the spinal cord after PI. Moreover, we found that oxycodone was able to reverse the increased expression of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), tyrosine kinase receptor (TrK) A, and TrkB and the decreased expression of NT-3 and TrkC, after PI. Pretreatment with oxycodone also altered the expression of these mediators.
Conclusion: Based on the results, possible underlying mechanisms for the antinociceptive properties of oxycodone in acute postoperative pain include the activation of MOR downstream signaling and the regulation of NTs and receptor expression through attenuation of glial activation and fortification of antinociceptive mediators in the spinal cord. This study may provide new insights into the molecular mechanisms underlying the analgesic action of oxycodone.
Keywords: oxycodone, acute postoperative pain, opioid receptors, neurotrophins, spinal cord