110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

纳武单抗治疗晚期非小细胞肺癌的荟萃分析
Authors Chen S, Hu B, Li H
Received 13 April 2018
Accepted for publication 17 July 2018
Published 31 October 2018 Volume 2018:11 Pages 7691—7697
DOI https://doi.org/10.2147/OTT.S171072
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Non-small-cell lung cancer (NSCLC) is often associated with rapid progression following standard chemotherapy. Nivolumab, an inhibitor of PD-1/PD-L1, is reported to have potential efficacy for the treatment NSCLC.
Objective: The purpose of this meta-analysis was to systematically evaluate the efficacy and safety of nivolumab in patients with advanced NSCLC.
Methods: Online electronic databases were searched in June 2017, including: PubMed, Embase, and the Cochrane Library. Randomized controlled trials were included that compared nivolumab to chemotherapy in NSCLC patients with regard to oncological outcome profiles. Review Manager Version 5.3 software was used.
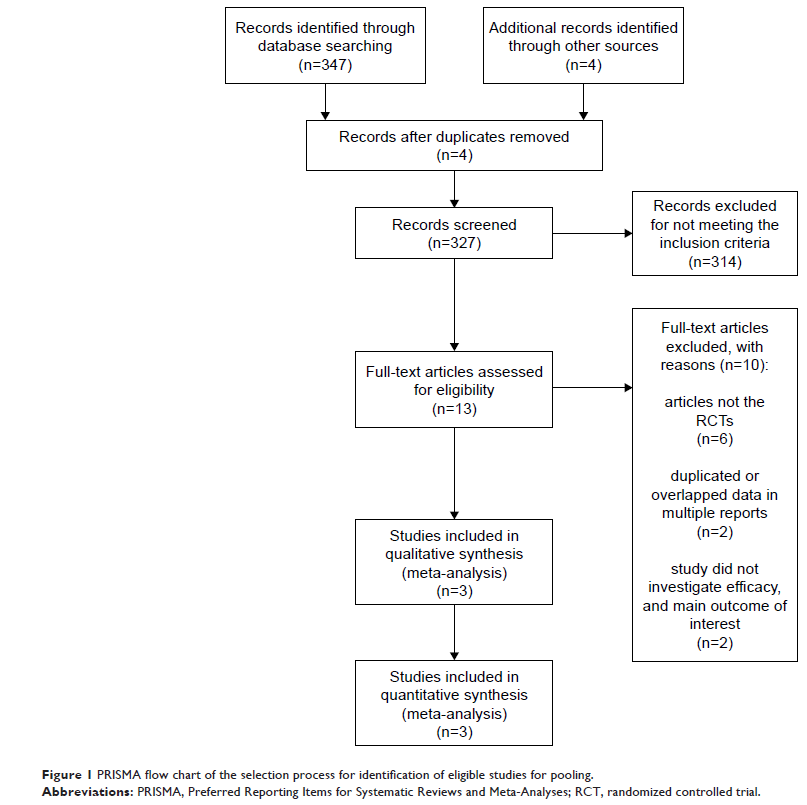
Results: Three studies were included in this analysis, comprising 1,395 patients with NSCLC, of whom 698 received nivolumab and 697 received chemotherapy without nivolumab. The pooled hazard ratios for overall survival (OS) and prolonged progression-free survival (PFS) were 0.77 (95% CI: 0.57–1.03; P =0.08) and 0.88 (95% CI: 0.64–1.20; P =0.41), respectively. The pooled odds ratio for overall response rate was 1.40 (95% CI: 0.66–2.96; P =0.39), indicating that no benefit with nivolumab was found for OS, PFS, or overall response rate. However, the odds ratio for treatment-related adverse events, grades 3 or 4, between the patients who received nivolumab and chemotherapy was 0.13 (95% CI: 0.09–0.17; P <0.00001). For patients with a PD-L1 expression level of 5% or more, no difference was observed in PFS (95% CI: 0.70–1.00; P =0.05) and OS benefit (95% CI: 0.34–1.15; P =0.13) between the groups.
Conclusion: These data demonstrate no clinical survival benefit with nivolumab for NSCLC patients, even in a subpopulation of patients with levels of PD-L1>5%. However, nivolumab had a more favorable safety profile than chemotherapy. Future investigations are needed to determine whether the efficacy of nivolumab can be improved.
Keywords: non-small-cell lung cancer, PD-1, nivolumab, meta-analysis