110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

单剂量和多剂量罗氟司特的药代动力学:一项针对健康中国志愿者的开放式三向交叉研究
Authors Huang J, Fu CX, Yang XY, Cui C, Yang S, Kuang Y, Guo CX, Hu P, Pei Q, Yang GP
Received 2 July 2018
Accepted for publication 22 October 2018
Published 26 November 2018 Volume 2018:12 Pages 4047—4057
DOI https://doi.org/10.2147/DDDT.S178862
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Purpose: To
determine the pharmacokinetic properties of the common tablet of roflumilast
administered in single and multiple oral doses in Chinese subjects.
Subjects and methods: Both the
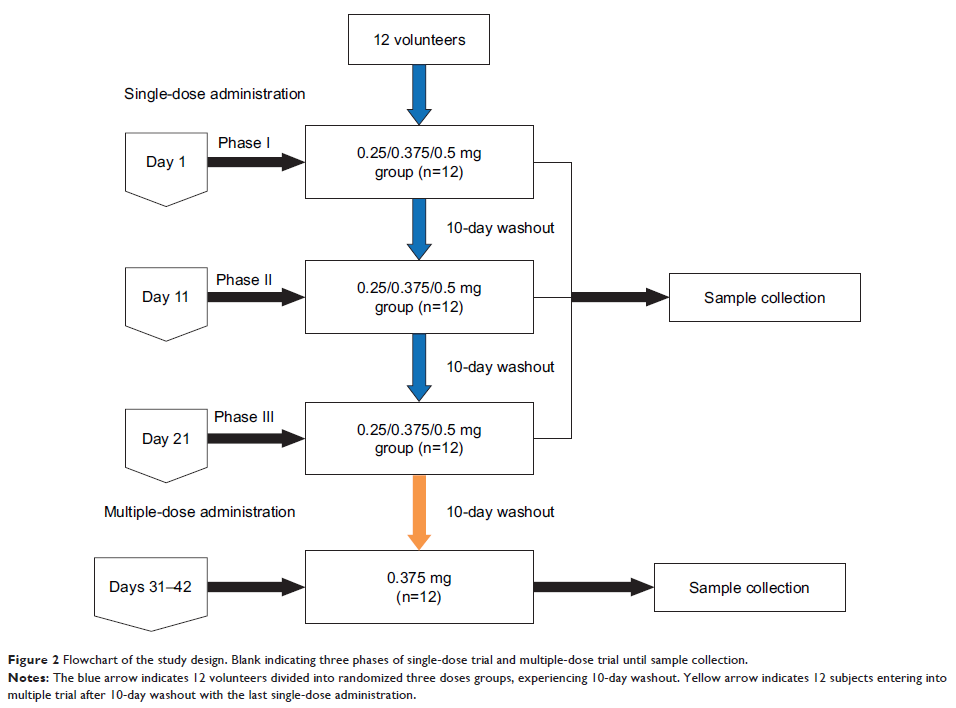
single- and multiple-dose studies included 12 adults (6 males and 6 females).
In this single-center, open-label study, single doses of 0.25, 0.375, and 0.5
mg were administered using a randomized, three-way crossover design, and then,
the 0.375 mg dose was continued for 11 days once daily. The pharmacokinetic
parameters for roflumilast and roflumilast N -oxide were
determined and the safety evaluation included adverse events assessed by
monitoring, physical examination, vital sign tests, and clinical laboratory
tests.
Results: After
every single dose, the time to the maximum concentration (C max) of
roflumilast (T max) was 0.25–2.0
hours; thereafter, the concentration declined, with a mean half-life (t 1/2) of 19.7–20.9
hours over the range of 0.25–0.50 mg. As for roflumilast N -oxide, the
mean t 1/2 was
23.2–26.2 hours. The area under curve from the beginning to 24 hours (AUC 0–24 h), the AUC
until infinity (AUC inf), and
the C max of
roflumilast and roflumilast N -oxide increased in a dose-proportional manner. After
multiple doses, the accumulation index (Rac) on the 11th
day of the steady state was ~1.63 for roflumilast and 3.20 for
roflumilast N -oxide. No significant sex differences were observed
in the pharmacokinetic parameters of roflumilast and roflumilast N -oxide. In
addition, there were no serious adverse events across the trial.
Conclusion: Roflumilast
was safe and well-tolerated in healthy volunteers, and a linear increase in
its C max and AUC
values was observed at doses ranging from 0.25 to 0.50 mg.
Keywords: pharmacokinetics,
roflumilast, roflumilast N-oxide, healthy volunteer, phosphodiesterase 4
inhibitor