110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

顶端靶向的口腔胶束表现出高效的肠道摄取和口服吸收
Authors Wang JL, Wang LF, Li Y, Wang XH, Tu PF
Received 13 August 2018
Accepted for publication 9 October 2018
Published 26 November 2018 Volume 2018:13 Pages 7997—8012
DOI https://doi.org/10.2147/IJN.S183796
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Yu Mi
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Introduction: Polymeric
micelles (PMs) hold promise for improving solubility and oral absorption of
poorly soluble drugs. Unfortunately, the oral absorption of PMs is also limited
by intestinal epithelium. To improve the oral delivery efficiency of micelles,
transporter-mediated micelles could enhance the transport efficiency across the
epithelial barrier, and they have attracted more attention.
Methods: Peptide
transporter 1 (PepT1)-mediated micelles (Val-PMs/Phe-PMs) were designed by
grafting valine (or phenylalanine) onto the surface of curcumin
(Cur)-loaded-D-α-tocopheryl polyethylene glycol 1000 succinate micelles
(TP-PMs). The oral absorption mechanism and oral bioavailability were further
investigated in vitro and in vivo.
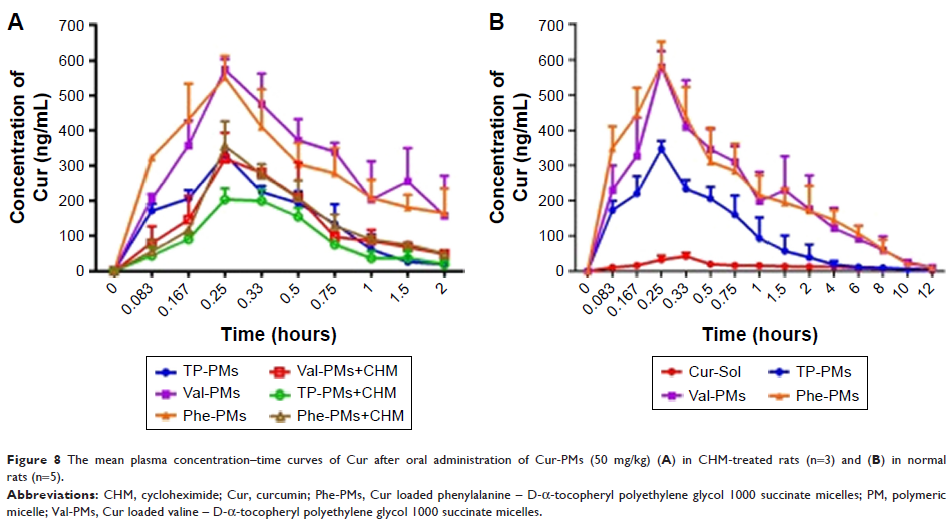
Results: The
cellular study showed that Val-PMs/Phe-PMs had a high PepT1 affinity, resulting
in a higher drug uptake and transcellular transport than TP-PMs. In rats,
Val-PMs/Phe-PMs exhibited higher intestinal accumulation in the apical side of
the intestinal epithelium than TP-PMs, promoting drug diffusion across
epithelial barrier. The oral bioavailability of Cur was significantly improved
by Val-PMs/Phe-PMs, which was about 10.50- and 3.40-fold greater than that of
Cur-Sol and TP-PMs, respectively.
Conclusion: PepT-1-mediated
micelles, using PepT1 as a target on intestinal epithelium, have unique
functions with intestine and prove promising for oral delivery of poorly
water-soluble drugs.
Keywords: PepT1,
micelles, epithelial barrier, curcumin, oral delivery