110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

胸腔内注射贝伐单抗加培美曲塞治疗恶性胸腔积液诱导的非鳞状非小细胞肺癌疗效较好
Authors Song X, Chen D, Guo J, Kong L, Wang H, Wang Z
Received 15 August 2018
Accepted for publication 23 October 2018
Published 27 November 2018 Volume 2018:11 Pages 8421—8426
DOI https://doi.org/10.2147/OTT.S184030
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Background and objective: Several
clinical trials have reported that intrapleural infusion of bevacizumab with or
without cisplatin exhibits encouraging efficacy in nonsquamous non-small cell
lung cancer (NS-NSCLC) patients with malignant serous cavity effusion. However,
most of the studies included a number of different types of cancers or
different hydrops types rather than focusing on one. In addition, no study
reported the efficacy and toxicity of intrapleural infusion of bevacizumab and
pemetrexed for advanced NS-NSCLC patients with malignant pleural effusion (MPE).
Patients and methods: We
retrospectively collected patients with MPE mediated from NS-NSCLC who
underwent intrapleural infusion of bevacizumab between August 2012 and February
2017. According to the different combined agents with bevacizumab, we divide
patients into two groups: Group 1 (BP Group) intrapleural infusion of
bevacizumab combined with pemetrexed and Group 2 (BD group) intrapleural
infusion of bevacizumab combined with cisplatin.
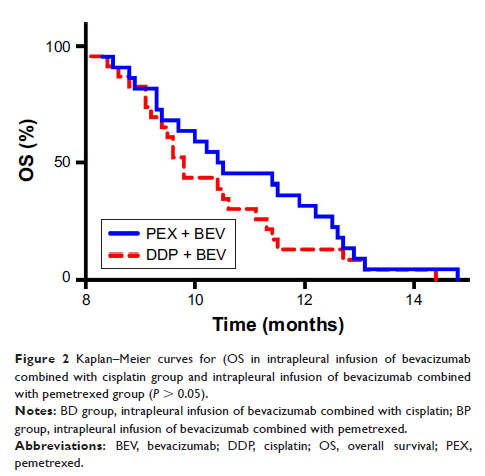
Results: A total
of 45 patients were enrolled in this study. Twenty-two of them received
intrapleuralinfusion of bevacizumab and pemetrexed every 2 weeks, 23 received
bevacizumab and cisplatin after draining effusion as much as possible. The
progression-free survival for patients in BP group was significantly higher
than BD group (P <
0.05) while the overall survival between the two groups was not significantly
different (P >
0.05). In addition, there was no statistical difference in adverse effects
between two groups.
Conclusion: Intrapleural
infusion of bevacizumab and pemetrexed is effective and tolerable for patients
with MPE mediated from NSCLC.
Keywords: NS-NSCLC,
MPE, bevacizumab, pemetrexed