110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Activation of polymeric nanoparticle intracellular targeting overcomes chemodrug resistance in human primary patient breast cancer cells
Authors Abou-El-Naga AM, Mutawa G, El-Sherbiny IM, Mousa SA
Received 1 August 2018
Accepted for publication 26 September 2018
Published 29 November 2018 Volume 2018:13 Pages 8153—8164
DOI https://doi.org/10.2147/IJN.S182184
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Thomas J. Webster
Background: Successfully overcoming obstacles due to
anticancer drugs’ toxicity and achieving effective treatment using unique
nanotechnology is challenging. The complex nature of breast tumors is mainly
due to chemoresistance. Successful docetaxel (DTX) delivery by nanoparticles
(NPs) through inhibition of multidrug resistance (MDR) can be a bridge to
enhance intracellular dose and achieve higher cytotoxicity for cancer cells.
Purpose: This
study tested primary patient breast cancer cells in vitro with traditional free
DTX in comparison with polymeric nanocarriers based on poly lactic co-glycolic
acid (PLGA) NPs.
Materials and methods: Establishment of primary cell line from breast malignant tumor
depends on enzymatic digestion. Designed DTX-loaded PLGA NPs were prepared with
a solvent evaporation method; one design was supported by the use of folic acid
(FA) conjugated to PLGA. The physical properties of NPs were characterized as
size, charge potential, surface morphology, DTX loading, and encapsulation
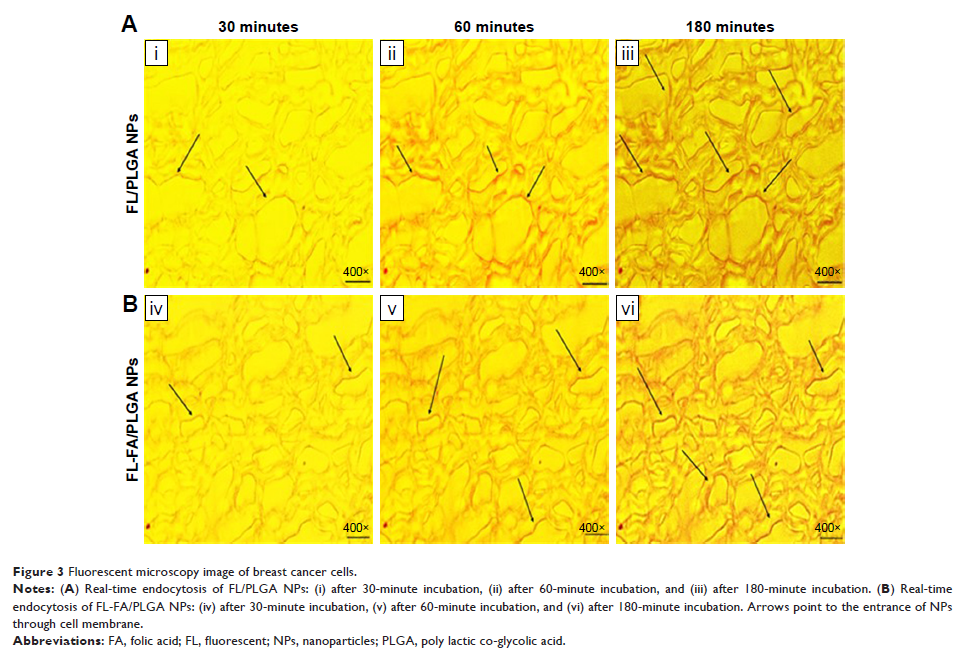
efficiency. In vitro cellular uptake of fluorescent NPs was examined visually
with confocal fluorescence microscopy and quantitatively with flow cytometry.
In vitro cytotoxicity of all DTX designed NPs against cancer cells was
investigated with MTT assay. RT-PCR measurements were done to examine the
expression of chemoresistant and apoptotic genes of the tested DTX NPs.
Results: Cellular
uptake of DTX was time dependent and reached the maximum after loading on PLGA
NPs and with FA incorporation, which activated the endocytosis mechanism. MTT
assay revealed significant higher cytotoxicity of DTX-loaded FA/PLGA NPs with
higher reduction of IC50 (8.29 nM). In addition, PLGA NPs, especially FA
incorporated, limited DTX efflux by reducing expression of ABCG2 (3.2-fold)
and MDR1 (2.86-fold),
which were highly activated by free DTX. DTX-loaded FA/PLGA NPs showed the
highest apoptotic effect through the activation of Caspase-9, Caspase-3, and
TP53 genes by 2.8-, 1.6-, and 1.86-fold, respectively.
Conclusion: FA/PLGA
NPs could be a hopeful drug delivery system for DTX in breast cancer treatment.
Keywords: PLGA
NPs, chemoresistance, endocytosis, drug delivery system, active targeting,
human breast cancer, DTX loaded PLGA NPs